Article Text
Abstract
Background Cognitive–behavioural therapy for insomnia (CBTi) is the first-line treatment for those with this sleep disorder. However, depressive and anxiety symptoms often co-occur with acute insomnia, which may affect the effectiveness of CBTi treatment.
Aims This study aimed to determine the impact of depressive and anxiety symptoms on the efficacy of CBTi in treating acute insomnia.
Methods A single-arm clinical trial was conducted among individuals who have acute insomnia. Participants underwent self-guided CBTi for 1-week. Their insomnia, depressive symptoms and anxiety symptoms were evaluated using the Insomnia Severity Index and the Hospital Anxiety and Depression Scale at baseline, post-treatment and 3-month follow-up. Repeated measures analysis of variance was used to assess the effectiveness of CBTi in treating insomnia, depressive symptoms and anxiety symptoms. A multivariate Cox regression model was used to determine the impact of depressive and anxiety symptoms on insomnia.
Results The study found significant reductions in insomnia, depressive symptoms and anxiety symptoms at both post-treatment and 3-month follow-up (F=17.45, p<0.001; F=36.37, p=0.001; and F=81.51, p<0.001, respectively). The duration of CBTi treatment had a positive impact on insomnia recovery (hazard ratio (HR)=0.94, p=0.018). However, baseline depressive symptoms (HR=1.83, p=0.004) and baseline anxiety symptoms (HR=1.99, p=0.001) had significant negative effects on insomnia recovery.
Conclusions The study showed that a 1-week self-guided CBTi treatment is effective in treating acute insomnia and comorbid depressive and anxiety symptoms. However, baseline depressive and anxiety symptoms negatively impact treatment effectiveness. Therefore, clinicians should assess for depressive and anxiety symptoms before treating acute insomnia with monotherapy CBTi.
- depression
- anxiety
- COVID-19
- cognitive behavioral therapy
- risk factors
Data availability statement
Data are available upon reasonable request. Data (deidentified participant data) are available upon reasonable request from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Cognitive–behavioural therapy for insomnia (CBTi) is the first-line treatment for insomnia. The symptoms of depression and anxiety are highly comorbid with insomnia, which may impact the effectiveness of CBTi.
WHAT THIS STUDY ADDS
We confirmed that baseline depressive and anxiety symptoms impaired the efficiency of CBTi when treating acute insomnia.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study suggests that clinicians should evaluate symptoms of depression and anxiety before providing monotherapy CBTi for acute insomnia.
Introduction
Acute insomnia is characterised by the persistence of insomnia symptoms for a brief period of time before diagnosis, typically lasting one to several days or up to a few weeks.1 People with acute insomnia experience significantly higher levels of psychological distress.1 Situational insomnia, also known as acute insomnia, has become more prevalent among people in hospitals and communities under the high and continuous stress of coronavirus disease 2019 (COVID-19)2 3 and can potentially develop into chronic insomnia following repeated episodes.4 The recommended first-line treatment for insomnia is cognitive–behavioural therapy for insomnia (CBTi), which includes multiple components such as stimulus control techniques, sleep education, cognitive therapy and sleep restriction techniques.5 During the COVID-19 pandemic, CBTi through telehealth became more available, showing comparable efficacy to traditional face-to-face treatment.6 Our team found that a 1-week regiment of self-guided CBTi was effective for treating acute insomnia during the COVID-19 pandemic.2 Most studies on the efficacy of CBTi have focused on treatment methods (eg, face-to-face vs telehealth), but few studies have examined the impact of insomnia comorbidity.
Insomnia, depression and anxiety are highly prevalent and are frequently comorbid, with individuals who have insomnia being five times more likely to experience depression or anxiety.7 Our team has reported that depressive and anxiety symptoms commonly co-occur with acute insomnia in the community.2 The comorbidities of insomnia, depression and anxiety may lead to more severe night-time, functional and quality-of-life impairments and confuse clinicians with more difficult diagnoses and treatment strategies.8 Given the high comorbidity of insomnia, depression and anxiety, and the current limitations in treating depression and anxiety, more research is being conducted to use CBTi to treat depression and anxiety when comorbid with insomnia.7 Symptoms of depression and anxiety are reported to be the strongest predictors of decreased insomnia response and remission after treatment, which may impair the efficacy of CBTi. Previous studies have explored the interaction between insomnia and depressive/anxiety symptoms during CBTi, but the results have been inconsistent,8 9 with some studies reporting positive effects from CBTi on symptoms of depression and anxiety,10 11 while other studies have found that the comorbidities of depression and anxiety may reduce the efficiency of CBTi for insomnia treatment.12 13
The present study aimed to determine symptoms of depression and anxiety and their impact on the efficacy of CBTi in treating acute insomnia. We hypothesised that the presence of depression and anxiety symptoms was related to the treatment effect. Identifying significant predictors of treatment effectiveness may guide the selection of treatment types and contribute to the development and implementation of new strategies and management for CBTi in the treatment of acute insomnia in the future.
Methods
Participants
A single-arm clinical trial was conducted. This article extends the findings from a campaign called ‘Prevention and Protection Handbook Against the Epidemic’ that was supported by the Guangzhou government in China to evaluate the efficacy of a 1-week self-guided CBTi regimen for acute insomnia among community adults.2 Refer to our earlier study for additional details of the campaign, the population studied, and the methodology.2 Participants who met the following criteria were included: (1) insomnia criteria according to DSM-V; (2) Insomnia Severity Index (ISI) score of 8 or higher; (3) insomnia duration of <1 month; and (4) age of 18 years or older. Exclusion criteria included being unable to understand the questionnaire, having an ISI < 8, and having insomnia for more than 1 month.
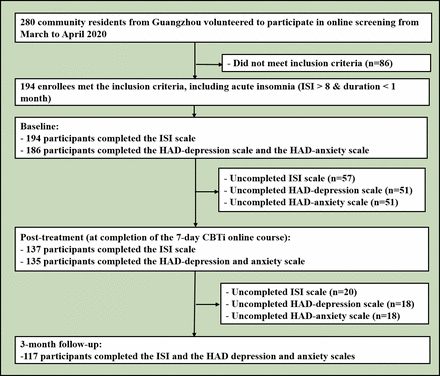
Assessment methods included evaluations of depressive/anxiety symptoms with the Hospital Anxiety and Depression Scale (HADS)14 and insomnia with the ISI scale,15 which were reported in our previous work.2 From March to April 2020, a total of 280 community participants volunteered for online screening, and 194 met the inclusion criteria (See flowchart in figure 1). All participants were assessed online at baseline, after 1 week of intervention using CBTi and at a 3-month follow-up. At baseline, among the eligible individuals with acute insomnia (n=194), all (100%) participants completed the ISI scale and 186 (95.9%) completed the HADS. At the 1 week completion of the course, 137 (70.6%) participants completed the ISI scale, while 135 (72.6%) completed the HADS. At the 3-month follow-up, 117 participants completed the ISI scale (85.4%) and the same number for the HADS (86.7%).
Flowchart of the study. HAD, Hospital Anxiety and Depression; ISI, Insomnia Severity Index.
CBTi intervention
The 1-week online CBTi treatment was supported by the WeChat platform, which is commonly used in China. The seven modules, as described previously (~15 min per module), consisted of sleep hygiene and sleep education (first day), sleep restriction (second day), stimulation control (third day), relaxation training (fourth day), cognitive restructuring (fifth day), core beliefs about sleep medicine (sixth day), and review and summary (seventh day).2
Statistical analysis
Analyses of the data were conducted using SPSS V.20 for Windows. The changes in depressive/anxiety/insomnia symptoms were performed with unequal time interval repeated measures analysis of variance (baseline=1, post-treatment=7 and 3-month follow-up=90). Contrast analyses were performed when there were significant time effects. Then, a survival analysis (event=insomnia, time=baseline, post-treatment and 3-month follow-up) was performed to examine the associations between insomnia and depressive/anxiety symptoms. The diagnosis of insomnia was defined as an ISI cut-off score of ≥8, while depressive and anxiety symptoms set the cut-off score at ≥11.16 17 Variables of depressive symptoms were categorised into four categories: no depressive symptoms=0, baseline depressive symptoms=1, depressive symptoms after treatment=2, and depressive symptoms at the 3-month follow-up=3. Anxiety symptoms were categorised in the same way. Insomnia-free survival was calculated among three time points, including baseline, after treatment and at 3-month follow-up, with adjustment for age, gender, body mass index (BMI) and treatment duration (0–7 days). The statistical significance level was set at a p value of <0.05.
Results
Demographic and clinical characteristics
Of the 280 volunteer community participants, 194 met the criteria for inclusion in the study. The majority of individuals with acute insomnia were young adults (37.11 (10.77)), female (70.1%), had normal BMI (21.92 (3.06)), had a relatively higher education level (15.48 (3.03)), were married (62.9%), were living with children (44.3%) and had full-time employment (76.8%). The sleep latency distribution was relatively even (29.9% were 11–30 min, 30.4% were 31–60 min and 34.5% were >60 min). Their mean sleep duration was 6.28 (3.08) hours. The participants' average attendance during the 7-day CBTi intervention was 4.67 (2.43) days (table 1).
Characteristics of study participants at baseline assessment (n=194)
Changes in depressive/anxiety symptoms and insomnia from baseline to 3-month follow-up
Table 2 shows depressive/anxiety symptoms and insomnia at baseline, post-treatment and at 3-month follow-up. The mean scores were 7.68 (4.17) and 8.15 (4.24) for depressive and anxiety symptoms, respectively, at baseline. For insomnia, the mean score was 14.70 (4.88) on the ISI. Significant reductions were found at post-treatment and at 3-month follow-up, including depressive and anxiety symptoms and insomnia.
Changes in depressive, anxiety and insomnia symptoms and their differences at baseline, post-treatment and 3-month follow-up
There were 194 (100%) participants diagnosed with insomnia using the ISI scale at baseline, 74 (54.0%) at post-treatment (30 had recovered at the 3-month follow-up; 44 had not), and 46 (39.3%) at the 3-month follow-up (44 had not recovered; 2 had recovered at post-treatment but were re-diagnosed at the 3-month follow-up). The numbers of participants with depressive symptoms were 48 (25.8%) at baseline, 7 (5.2%, including 3 continuous and 4 newly diagnosed with depressive symptoms) at post-treatment and none at the 3-month follow-up. For anxiety, there were 51 (27.4%) participants at baseline, 16 (11.9%, including 8 continuous and 8 with new anxiety symptoms) at post-treatment and none at the 3-month follow-up.
Impact of depressive and anxiety symptoms on acute insomnia treatment with CBTi
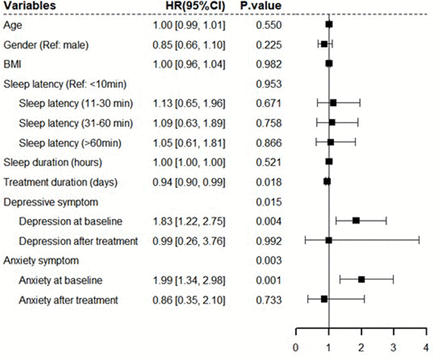
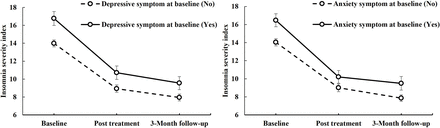
A forest plot showed multivariate Cox regression analysis of the effect of depressive/anxiety symptoms on insomnia survival (figure 2). Baseline depressive symptoms (HR=1.83, p=0.004, 95% CI: 1.22 to 2.75) and baseline anxiety symptoms (HR=1.99, p=0.001, 95% CI: 1.34 to 2.98) had significant negative effects on insomnia recovery (figure 2). The CBTi treatment duration had a positive impact on insomnia (HR=0.94, p=0.018, 95% CI: 0.90 to 0.99) (figure 2). Participants with baseline depressive or anxiety symptoms showed more severe insomnia and less benefit from CBTi treatment than those without these comorbidities (figure 3). Figure 3 shows the changes in ISI by baseline depressive and anxiety symptom categories (yes/no).
Forest plot of multivariate Cox regression model of insomnia. BMI, body mass index. CI, confidence interval; HR, hazard ratio.
Changes in Insomnia Severity Index with baseline depressive and anxiety symptom categories (yes/no) at different time points. CBTi, cognitive–behavioural therapy for insomnia.
Discussion
Main findings
Our study suggests that 1 week of CBTi can effectively treat acute insomnia and comorbid symptoms of depression and anxiety. Moreover, individuals with better adherence to the CBTi treatment showed better efficacy. However, our findings also indicate that baseline symptoms of depression and anxiety may negatively impact the effectiveness of CBTi in treating acute insomnia, especially in the context of a pandemic. These results emphasise the significance of addressing depressive and anxiety symptoms in the management of insomnia with CBTi treatment. Our study can aid clinicians in refining their CBTi treatment strategies and improving the management of acute insomnia with comorbid depressive and anxiety symptoms.
Our team found that a longer, 1-week self-guided CBTi treatment leads to better treatment efficiency for acute insomnia. This indicates that CBTi has a significant effect on acute insomnia. Our previous work has confirmed that completing the entire CBTi treatment for 7 days was more beneficial for individuals who did so than those who did not.2 However, in the present study, only 40.8% of participants completed the entire daily treatment (7 days) of the 1-week self-guided CBTi. This reflects the challenge of achieving compliance with CBTi among patients with insomnia. Previous studies have shown that compliance with CBTi can be challenging for clinicians and behavioural therapists, especially when working with those with chronic insomnia.18 For example, the withdrawal rate for chronic insomnia was 33% in an online CBTi treatment with a frequency of five times a week.19 Traditional or classical CBTi treatment for chronic insomnia takes 6–8 weeks, but this lengthy duration can lead to high withdrawal rates. To improve treatment adherence, we shortened the duration of CBTi treatment but found it was unhelpful in increasing compliance. Significantly, our results were contrary to our expectations; our study reflected poorer compliance with CBTi treatment when the frequency was higher (daily vs weekly) and the duration was shorter (1 week vs 5 weeks).19 One possible reason is that treatment frequency is more harmful to CBTi treatment compliance than treatment duration. Future studies should clarify the relationships between treatment duration, treatment frequency and treatment compliance with CBTi for insomnia to understand better how to improve patient adherence to CBTi.
CBTi treatment has been found to improve symptoms of depression in multiple studies,20–24 as well as symptoms of anxiety,25 26 which makes this study consistent with previous research. In addition, symptoms of depression and anxiety had disappeared at the 3-month follow-up, which is in line with a previous study.9 Improved sleep quality following CBTi treatment may be one possible reason for the reduction in depressive and anxiety symptoms. Another possibility is that some of the CBTi elements, such as relaxation exercises, can relieve negative emotional symptoms by reducing cognitive arousal and sleep latency, which may, in turn, reduce comorbidities associated with anxiety symptoms.
The effectiveness of CBTi treatment for acute insomnia is negatively impacted by both baseline depressive and anxiety symptoms. These findings are consistent with previous studies that have identified depressive symptoms12 27 and anxiety symptoms28 as risk factors for the efficiency of CBTi treatment. One retrospective study evaluated the effects of depression and anxiety symptoms on insomnia treatment and found no effect, which is inconsistent with our study.9 Another study grouped participants based on low/high depression and found no effect of depression on CBTi treatment.29 There are several possible reasons for these discrepancies. First, the present study evaluated changes in depressive and anxiety symptoms at baseline, post-treatment and 3-month follow-up with continuous variable data, while other studies categorised symptoms as minor/mild/severe or low/high.9 ,29 Second, the duration of insomnia may also differ between studies, with our study focusing on acute insomnia and other studies focusing on chronic insomnia.9 29 Additionally, the severity of baseline insomnia and symptoms of depression and anxiety may also be important factors that impact the effectiveness of CBTi treatment. In our study, the mean scores for the ISI and HAD-depression/anxity evaluations were 14.70 and 7.7/8.2, respectively, compared with 19.4 and 12.3/7.6 in another study.10 Based on the standardised cut-off value, 25.8% of the participants had moderate to severe depressive symptoms, while 27.4% had at least moderate anxiety symptoms at baseline.10 The severity of insomnia in our community population setting was relatively lower than that seen in other earlier study.9 This could be due to differences in study populations. Our study focused on providing CBTi to individuals with acute insomnia in the community, which may also contribute to the discrepancies in prevalence rates. Another study found no significant effect of depressive and anxiety symptoms on insomnia comorbid with sleep apnoea.30 However, how the complex relationship between insomnia and sleep apnoea impacts the effectiveness of CBTi needs to be considered. It is worth noting that depressive symptoms have been reported to increase the risk of future insomnia.31
Limitations
Our study has several limitations. First, we did not include a control group; however, this did not affect the main aim of our research, which was to investigate the effect of depression and anxiety symptoms on changes in insomnia during CBTi treatment. Second, while using reliable and valid symptom severity scales was helpful, we did not measure diagnoses of major depressive disorder or general anxiety disorder before or after treatment. Third, it is important to note that our study focused on using a 1-week online self-guided CBTi treatment for acute insomnia. It may not apply to chronic insomnia due to the longer course and more complex causes associated with that condition.
Implications
Our study confirms the efficacy of a one-week self-guided CBTi treatment in managing acute insomnia, as well as its comorbid symptoms of depression and anxiety. However, we found that both baseline depressive and anxiety symptoms can negatively impact treatment effectiveness. In fact, depressive symptoms were found to increase the risk of future insomnia, which can result in the conversion from acute to chronic insomnia.31 Therefore, it is crucial for clinicians to re-evaluate the use of CBTi monotherapy and allow treatment in the presence of comorbid symptoms of depression and anxiety.32 33 Prior to initiating CBTi treatment for acute insomnia, it is necessary to address any underlying symptoms of depression and anxiety. It is worth noting that the prevalence of insomnia in older patients is high, especially during the COVID-19 pandemic.34 35 Our online CBTi treatment may not be appropriate for this population due to their limited mobile phone use. In future research, we recommend using structured instruments to assess depressive and anxiety symptoms before and after CBTi treatment and investigating specific strategies that can help reduce these symptoms. Additionally, further research should explore the effectiveness of CBTi in treating and preventing comorbidities such as depression and anxiety.
Data availability statement
Data are available upon reasonable request. Data (deidentified participant data) are available upon reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Nanfang Hospital, Southern Medical University (ethics approval number not applicable). The participants gave informed consent to participate in the study before taking part.
References
Chenxi Zhang obtained her bachelor’s degree in clinical medicine from Guangdong Medical College, China in 2011. She obtained a master’s degree from the Second Xiangya Hospital of Central South University in China in 2014 and a PhD degree from the Chinese University of Hong Kong in 2018. She is currently working as a postdoctoral fellow in the Department of Psychiatry, Nanfang Hospital of Southern Medical University since June 2022. She has participated in research related to CBTi interventions for insomnia as part of the National Key R&D Program of China. She has also over seen a project for the Guangzhou Basic Research Program. Her main research interests include sleep disorders and CBTi treatment.

Footnotes
Contributors CZ wrote the first draft of the manuscript and conducted the statistical analyses. SZ, YX, SL, SD, ZL, LF and LZ interviewed the participants and organised the primary data. CZ and BZ designed the study and provided supervision in the implementation of the study. BZ was responsible for the overall content as the guarantor. All authors approved the final manuscript.
Funding This work was supported by the National Key R&D Program of China (BZ: grant number 2021YFC2501500), the Basic and Applied Basic Research Project of Guangzhou Basic Research Program (CZ: grant number 202201011502), the National Natural Science Foundation of China (BZ: grant number 82271525), Nanfang Hospital Clinical Research Project of Southern Medical University (BZ: grant number 2021CR009) and the Education Research Projects of Nanfang Hospital (BZ: grant number 21NJ-ZDPY01).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.