Article Text
Abstract
Background Major depressive disorder (MDD) is a common psychiatric disease and a leading cause of disability worldwide. Handgrip strength (HGS) as an objective physical fitness test is a practical index for identifying many diseases. Previous studies drew different conclusions about the relationship between HGS and MDD.
Aims We aim to explore whether HGS has an effect on the risk of MDD.
Methods HGS-related single-nucleotide polymorphisms identified by a genome-wide association study were used as instrumental variables in this Mendelian randomisation (MR) study. Summary data on MDD were obtained from the Psychiatric Genomics Consortium. Four methods were applied, including inverse variance weighted (IVW), MR Egger, weighted median and weighted mode. Additional sensitivity analyses, including leave-one-out, heterogeneity test, pleiotropy test and confounders identification, were conducted to test the robustness of our results.
Results Each 1 kg increase in left HGS is associated with a 21.95% reduction in the risk of MDD (ORIVW = 0.781, 95% CI: 0.650 to 0.937, p=0.009), while no significant correlation exists in the estimation of right HGS (p=0.146). Sensitivity analyses demonstrated statistical significance (βIVW = −0.195, p=0.023) after excluding some genetic loci that cause pleiotropy.
Conclusions Increased left HGS is associated with a reduced risk of MDD. In the future, it may be used as an index for the clinical screening, observation and treatment of MDD.
- depression
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
It is necessary to find a suitable biomarker that can be an early predictor of the risk of major depressive disorder.
WHAT THIS STUDY ADDS
This work shows that increased handgrip strength is likely to indicate a lower risk of major depressive disorder.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
According to current outcomes, handgrip strength would be expected to become an indicator with suggestive significance for the risk of major depressive disorder.
Introduction
Major depressive disorder (MDD) is a common psychiatric condition that represents a leading cause of disability worldwide and is associated with deficits in cognition, including executive function and memory.1 These cognitive deficits impair social and occupational functioning, contributing to the personal, social and economic burden associated with mood disorders.2 However, preventing MDD has been challenging, with few established protective factors, particularly modifiable targets for prevention. Recent years witnessed the increasing recognition of the importance of objective physical fitness tests as indicators of physical, mental and cognitive outcomes in the general population.3 Handgrip strength (HGS) is quickly assessed and provides an objective measure of muscular fitness, emerging as an important biomarker of overall health and disease status. Assessing HGS asymmetry may provide insights into HGS, as one study has verified that handgrip asymmetry would hasten mortality.4 Owing to the strength of the evidence in this area, HGS is now considered an easily administered and clinically useful index of cognitive decline across the lifespan.3
Despite growing epidemiological evidence regarding the relationship between HGS and mental illness, studies using HGS or other objective physical performance measures to explore strength loss as an indicator of MDD and other common mental health conditions have yielded inconsistent results.5 6 Moreover, different dominant hands lead to different health outcomes; for example, different times to mortality were found in populations with a different dominant hand.4 Few studies have investigated lifestyle factors concerning MDD risk among people with normal HGS. Yet these results could be possibly biased by factors that influence both HGS and MDD. Consequently, findings of pre-existing observational studies are insufficient to draw a definitive conclusion on the causal relationship between HGS and MDD due to their susceptibility to reverse causality and potential confounders.
Mendelian randomisation (MR) analysis, in which genetic variants are used as instrumental variables (IVs), is a relatively precise epidemiological approach to evaluate the causal relationship between exposure and outcome with less susceptibility to potential confounding factors and reverse causality. Since genetic variations strictly adhere to the principle of random distribution at conception, they are generally independent of environmental risk factors, antecedent risk factors and disease development. One MR study has demonstrated that genetically predicted MDD was causally associated with an increasing risk of HGS decline.7 However, to date, there has been no report of the causality from HGS to MDD.
The aim of this study was to use population-scale data from large-scale genome-wide association studies (GWASs) to establish how muscular function measured by HGS relates to MDD and assess the impact of potential confounders on the outcome using a two-sample MR method. The findings, in turn, will improve our current understanding of the use of the handgrip dynamometer as an indicator of overall cognitive status, along with providing new insights into the contributing factors and possible interventions for cognitive functioning in people with and without psychiatric conditions.
Materials and methods
GWAS data of 634 094 subjects were included in this study. All selected data sets have obtained ethical approval.
Data sources of HGS and MDD
Depression severity of 59 851 patients with MDD and 113 154 healthy controls from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/) was considered as the outcome.8 A GWAS dataset of 461 089 individuals from the Medical Research Council-Integrative Epidemiology Unit (MRC-IEU) (http://www.bristol.ac.uk/integrative-epidemiology/) based on the UK Biobank9 was selected as the exposed group in our study. These data are available on request to approved researchers.
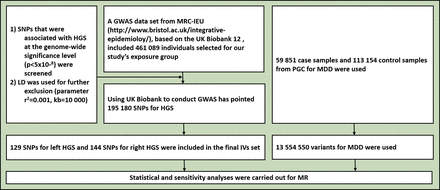
A previous GWAS based on the UK Biobank found that single-nucleotide polymorphisms (SNPs) explained approximately 23.9% of the variation in HGS across individuals.10 We screened out SNPs that were associated with maximal HGS at the genome-wide significance level (p<5×10−8) in the exposed group and used linkage disequilibrium (LD) for further exclusion (r2=0.001, kb=10 000). Finally, 129 SNPs associated with left HGS and 144 SNPs with right HGS were included in the instrument variants (IVs) set (figure 1, online supplemental table S1).
Supplemental material
Statistical analysis for MR
MR analyses were conducted with the ‘Two Sample MR’ package (V.0.5.6) in R studio (V.4.0.3). This package harmonises exposed and outcome datasets containing information on SNPs, effect alleles, other alleles, effect sizes (odds ratios (ORs) converted to beta statistics by log transformation), SEs, p values and effect allele frequencies for the selected exposure instruments. MR analysis was conducted using inverse-variance weighted (IVW), MR Egger, weighted median and weighted mode.
Sensitivity analysis for MR
Notably, in order to have a valid interpretation for the MR analysis, it is necessary to hold the following assumptions11 (figure 1):
The IVs are robustly correlated with HGS.
The IVs influence MDD only through their effect on HGS.
The IVs are independent of any confounders of HGS-MDD relation.
Flowchart for the enrolment. GWAS, genome-wide association study; HGS, handgrip strength; IVs, instrumental variables; LD, linkage disequilibrium; MDD, major depressive disorder; MR, Mendelian randomisation; MRC-IEU, Medical Research Council-Integrative Epidemiology Unit; PGC, Psychiatric Genomics Consortium; SNPs, single-nucleotide polymorphisms.
Sensitivity analyses examining whether these assumptions were satisfied were performed to confirm the robustness of our results. We conducted leave-one-out method, heterogeneity test and MR-Egger regression for horizontal pleiotropy12 and explored the potential confounders. These tests vary in principle but essentially capture the extent to which the effect for one or more instrument SNPs is exaggerated in magnitude, as would be the case if that SNP not only acted through the hypothesised pathway but through other unaccounted causal pathways.
The leave-one-out method was implemented to investigate whether the estimation of IVW analysis could be biased or determined by a particular SNP. It was performed based on the re-run IVW outcomes of the remaining SNPs after successively omitting one SNP each time. When the result is not affected by a specific SNP, the regression coefficients remain relatively stable.
The heterogeneity test was similar to that in the meta-analysis. Cochran’s Q test was used to examine the heterogeneity (p<0.05 was considered statistically significant).
In the MR-Egger regression, p<0.05 indicates the presence of horizontal pleiotropy. In addition, we used MR–Pleiotropy Residual Sum and Outlier (PRESSO) for further analysis.
To verify the third assumption, we employed additional MR analysis to investigate whether genetic predisposition towards HGS is associated with the potential confounders underlying the affecting factors of HGS. For instance, more alcohol consumption is related to both reduced HGS13 and increased risk of MDD.14 Larger waist circumference was frequently found in patients with MDD, compared with the general population.15 In addition, an independent negative association was found between HGS and metabolism-related markers, including waist circumference, triglycerides, high-density lipoprotein cholesterol (HDL-C), glucose and blood pressure (BP).16 Higher levels of triglycerides and lower levels of HDL-C were observed in case–control studies and meta-analyses.17 Glucose metabolism is an important pathway and mechanism for brain activities associated with MDD.18 Genes associated with blood pressure have genetic overlap with MDD.19 Overall, metabolism-related markers are considered potential confounders of the HGS–MDD relationship. Also, exercise and frailty are important confounders which are associated with HGS and MDD.20 21
Conventional MR was applied to investigate whether genetically predicted HGS was associated with these confounding factors or mediators. Data on alcohol assumption were obtained from the GWAS and Sequencing Consortium of Alcohol and Nicotine use, while genetic summary data on waist circumference were extracted from the Genetic Investigation of ANthropometric Traits (GIANT) (http://giant.princeton.edu/). Genetic instruments of triglycerides and HDL-C were obtained from 21 545 individuals in 14 cohorts of Europeans and the UK Household Longitudinal Study (http://understandingsociety.ac.uk). In addition, a data set of glucose was chosen from the published GWAS in the Meta-Analysis of Glucose and Insulin related traits Consortium (MAGIC) (https://magicinvestigators.org/), and information about blood pressure came from the International Consortium of Blood Pressure (ICBP). Data on exercise and frailty were obtained respectively from 350 492 Europeans recruited by Klimentidis et al and 175 226 Europeans by Atkins et al. (table 1).
Extra Mendelian randomisation analysis to explore confounders of the HGS–MDD relationship
Results
Causal effect between HGS and MDD
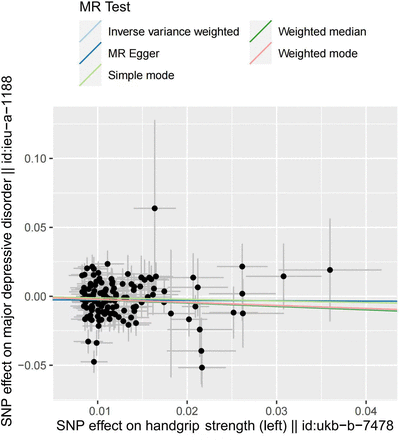
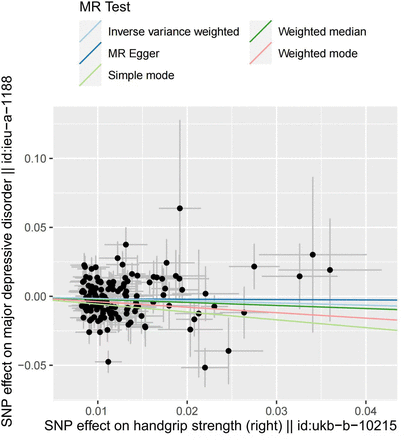
One-kilogram increase in left HGS was associated with a 21.95% reduction of risk for MDD (ORIVW=0.781, 95% CI: 0.650 to 0.937, p=0.009). No significant correlation was found in the estimation of right HGS (p=0.146). In addition, MR Egger showed ORleft handgrip = 0.997 (p=0.986), ORright handgrip = 0.984 (p=0.962). Weighted median showed OR left handgrip = 0.783 (p=0.032), ORright handgrip = 0.797 (p=0.038). Weighted mode showed OR left handgrip = 0.770 (p=0.434), ORright handgrip = 1.488 (p=0.130).
We recalculated the effect after excluding the overlapping SNPs between left and right handgrip, and the result again suggested that only the change of the left handgrip has an effect on MDD (left: ORIVW after elimination = 0.813, p=0.049; right: ORIVW after elimination = 0.915, p=0.386) (figures 2 and 3, online supplemental figures S1 and S2). Note that eliminating the overlaps may bias the result due to the potential violation of the first assumption of Mendelian randomisation study.
Supplemental material
Scatter plot of the effect of left handgrip strength on major depressive disorder. MR, Mendelian randomisation; SNP, single-nucleotide polymorphism.
Scatter plot of the effect of right handgrip strength on major depressive disorder. MR, Mendelian randomisation; SNP, single-nucleotide polymorphism.
Sensitivity analysis for the assessment of three MR assumptions and MR analysis
The first assumption was satisfied since all screening SNPs were associated with HGS at the genome-wide significance level (p<5×10−8), and the F-statistic was 1122 for left HGS and 1005 for right HGS (F>100) (online supplemental table S2). Furthermore, we assessed if the correlation between genetically predicted HGS and MDD was impacted by the aforementioned potential confounders, that is, alcohol drinks per day, waist circumference, triglycerides, HDL-C, glucose, BP, exercise and frailty. Results showed that, except for frailty (ORIVW right handgrip= 0.871, 95% CI: 0.779 to 0.974, p=0.016; ORIVW left handgrip= 0.882, 95% CI: 0.796 to 0.977, p=0.016), no significant correlation was found between genetically predicted HGS and potential confounders (table 2). On the genome-wide level, our genetic instrument of HGS-associated SNPs was not significantly associated with any other phenotype momentarily. In addition, the leave-one-out method showed no abnormal condition for left HGS while removing each SNP for right HGS, almost SNPs caused bias (online supplemental table S3, online supplemental figures S3 and S4). The MR-heterogeneity test showed the presence of heterogeneity (online supplemental table S4). Egger regression showed no obvious pleiotropy. Using MR-PRESSO for further discovery of pleiotropy, the result remained stable (β=−0.195, p=0.023) (online supplemental table S5). Funnel plots are also shown in the supplementary materials (online supplemental figures S5 and S6).
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Correlation between genetically predicted HGS and potential confounders
Discussion
Main findings
Using data from 461 089 participants for HGS and 173 005 subjects (59 851 cases and 113 154 controls) for MDD, our MR study demonstrated that genetically predicted increased left HGS was causally associated with a 21.95% lower risk of MDD. Furthermore, we observed that genetic predisposition towards HGS was not correlated with established potential risk factors for MDD, including alcohol drinks per day, waist circumference, triglycerides, HDL-C, glucose, exercise and BP.
Previous outcomes reported by observational studies on the relationship between HGS and MDD were inconsistent. A cohort study of 7364 subjects from Korea concluded that HGS was not associated with MDD in adults after adjustment for age, sex, exercise and so on (adjusted OR=1.116, 95% CI: 0.585 to 2.128, p=0.897).6 However, another meta-analysis and systematic review involving 33 030 participants found a strong correlation after controlling for covariances (adjusted OR=1.821, 95% CI: 1.160 to 2.590, p=0.002).22 Discrepant findings in previous work may result from the relatively limited sample sizes of patients with MDD, leading to insufficient statistical power to precisely evaluate causality between HGS and MDD risk. On the other hand, few studies managed to control confounders like waist circumference and alcoholic drinks per day.18 More importantly, no prospective large-scale longitudinal cohort study has been conducted to date. To address these problems, in this study we examined the causal relationship between HGS and MDD at the gene level after removing the interference of several confounding factors.
Implications
Special findings
Unexpectedly, we found that left HGS but not right HGS was associated with MDD. Among various factors that determine HGS, handedness may be the most important one. A previous study reported that left-handedness with a stronger left handgrip is associated with a 5.2% higher risk of being depressed.23 Handedness has been associated with brain lateralisation, which could be observed in patients with MDD.24 Moreover, a clinical trial using repetitive transcranial magnetic stimulation on 310 patients with MDD showed that targeting the left dorsolateral prefrontal cortex is more effective for left-handed than right-handed individuals.25 Left-handedness seems to be more clinically related to MDD.
Possible mechanisms
Potential mechanisms for increasing HGS on the reduced risk of MDD can be generally divided into two aspects. First, immunological reactions, including increased oxidative stress and inflammation, play an important role in their relationship. Oxidative stress, including reactive oxygen and nitrogen species (ROS/RNS), accumulates in the body as one ages and impairs muscle, causing the decline of HGS.26 ROS/RNS have also been related to MDD.27 Inflammatory factors, such as C reactive protein and interleukin-6, can both accelerate the catabolism of muscle28 and induce depressive symptoms.29 These biochemical processes affect movement and quality of life, indirectly causing low mood and even depressive symptoms. Second, HGS and MDD were both associated with hippocampal volume and white matter hyperintensities.30 Given that hippocampal volume reduction aggravates MDD in older depressed subjects, it is possible that decreased HGS correlates with cognitive dysfunction, a relevant symptom of MDD.30
Strength of the work
To the very best of our knowledge, this is the first MR study to assess the causality between genetically predicted HGS and MDD, with results highlighting the importance of HGS in the screening and clinical assessment of MDD. With large sample sizes (n=6 4 094) and robustly associated IVs, our two-sample MR provides adequate statistical power as well as a relatively precise estimation of a causal effect. Second, we performed additional MR analyses to investigate whether HGS-associated SNPs are linked to several confounding factors associated with the risk of MDD, namely, alcoholic drinks per day, waist circumference, triglycerides, HDL-C, glucose and BP. It also indicates that the causality between HGS and MDD was more likely due to the characteristics of HGS itself.
Limitations of this study
Several limitations in our study cannot be ignored. First, although we have used the most comprehensive set of genetic variants known to date, it merely explained a limited part of the HGS variation across individuals. Unknown HGS-related SNPs could also play an important role in the development of MDD. Second, all three MR assumptions cannot be fully examined in our study, and potential violations against the assumptions may occur. It is possible that some of the genetic variants were also associated with confounders of HGS and MDD in our study, and caution is needed when considering the gross effect. For instance, low grip strength is associated with impaired quality of life and is a recognised marker of frailty, predicting physical decline and functional limitations in daily life.21 Also, despite the genetic factors being estimated to account for about 23.9% of the variation, HGS is also influenced by environmental factors and its interaction with genes. Third, all participants in our study were European, and it remains unclear whether our finding applies to other populations.
In conclusion, our results indicated that people with higher HGS have a lower risk of MDD and that HGS may be useful in the screening and assessment of MDD. The association between HGS and MDD and its mechanism has not yet been fully investigated, thus warranting further exploration.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Nanxi Li is currently studying in the Mental Health College of Guangzhou Medical University. She has participated in the PsyNI Lab at the Affiliated Brain Hospital of Guangzhou Medical University. Her main research interests include the physiological mechanisms of brain imaging in mental diseases, such as repeated transcranial magnetic stimulation (rTMS) and magnetic resonance imaging (MRI). She conducts studies involving the interaction of brain science and genetics in the field of psychological medicine under the guidance of Professor Bin Zhang.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors NL is in charge of planning the study, conducting the work, writing first draft and revising it. RZ is responsible for conducting the work, modification and providing guidance of English. BZ is in charge of planning the study, modification and proofreading the manuscript. And, BZ is the guarantor who accepts full responsibility for the work and the conduct of the study.
Funding We received financial support from two sources: The National Natural Science Foundation of China (81801345) and the Planning Project of Guangdong Province of China (2019B030316001).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.