Article Text
Abstract
Background Depression in adolescents is recognised as a global public health concern, but little is known about the trajectory of its clinical symptoms and pathogenesis. Understanding the nature of adolescents with depression and identifying early biomarkers can facilitate personalised intervention and reduce disease burden.
Aims To track multidimensional outcomes of adolescents with depression and develop objective biomarkers for diagnosis, as well as response to treatment, prognosis and guidance for early identification and intervention.
Methods This is a multidimensional cohort study on the Symptomatic trajectory and Biomarkers of Early Adolescent Depression (sBEAD). We planned to recruit more than 1000 adolescents with depression and 300 healthy controls within 5 years. Multidimensional clinical presentations and objective indicators are collected at baseline, weeks 4, 8, 12 and 24, and years 1, 2, 3, 4 and 5.
Conclusions To the best of our knowledge, this is the first longitudinal cohort study that examines multidimensional clinical manifestations and multilevel objective markers in Chinese adolescents with depression. This study aims at providing early individualised interventions for young, depressed patients to reduce the burden of disease.
Trial registration number Chinese Clinical Trial Registry ID ChiCTR2100049066.
- depression
- adolescent psychiatry
- genetics, behavioural
- biological psychiatry
- cohort studies
Data availability statement
There are no data in this work.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
What is already known on this topic
Depression in adolescents is a heterogeneous and complicated disease with a lack of objective biomarkers.
What this study adds
We will identify distinct subtypes of adolescent depression by tracking the trajectory of clinical symptoms, diagnosis, and functional outcomes over a five-year follow-up period, ultimately creating highly homogeneous cohorts.
How this study might affect research, practice or policy
Our multidimensional time-dependent database will provide vital clues to identify the objective markers, which will ultimately promote the development of neurobiological-based diagnostic classification and early individualized interventions for adolescents with depression.
Introduction
Depression is a global public health problem that affects all ages.1 In recent years, the prevalence of depression in adolescents has exceeded that of adults.2 In the USA, the lifetime prevalence of a major depressive disorder (MDD) in adolescents is 11.0%, with a 12-month prevalence of 7.5%.3 According to the latest epidemiological data in China, the pointed prevalence of MDD in adolescents is 2%, higher than in previous surveys.4 Research suggests that depression in adolescents is associated with high recurrence rates, premature death, poor academic outcomes and psychosocial dysfunction, which may persist into adulthood or lead to other mental disorders,5 6 emphasising the need for early identification and intervention.
The biggest obstacle to early identification and intervention is the lack of objective markers of illness risk, progression, or response to treatment, although previous studies have explored biomarkers in genetics, neurobiology and gut flora, and so on.7 8 Lack of biomarkers leads to low treatment rates9 and high rates of misdiagnosis,1 especially in differentiating bipolar disorder from unipolar depression.10 The failure to resolve these issues, as well as the lack of combined multilevel units of biomarkers, may be due to the complexity and developmental heterogeneity of adolescents with depression. According to previous studies, one of the main sources of heterogeneity is clinical manifestations. The clinical characteristics of depression in adolescents differ from those of adults, and they manifest in more self-injury, suicidal thoughts and behaviours, comorbidity with other mental disorders and physical problems, sleep rhythm disturbances, and use of tobacco, alcohol or other substances.7 Another issue is the different patterns of disease persistence and clinical outcomes in adolescents with depression. About 30%–55% of individuals experiencing a depressive episode have subthreshold manic symptoms,11 12 while only 13%–33% of children and adolescents with depressive disorder may develop bipolar disorder.10 13 Thus, the age-dependent heterogeneity of depressive symptoms should be considered. In addition, adopting dimensional conceptualisations may help us understand the full spectrum of depression in adolescents better. Therefore, dividing the clinical presentation of research subjects into different dimensions based on the development of characteristics of symptoms, risk factors, and functional outcomes may provide important information regarding the ways by which psychopathology gradually emerges throughout development. Moreover, the combination of different biomarkers cutting across different levels of disease complexity may lead to the identification of objective biomarkers for adolescents with depression.14 Therefore, it is essential to conduct long-term longitudinal observation to capture the biopsychosocial profile for identifying accurate biomarkers.
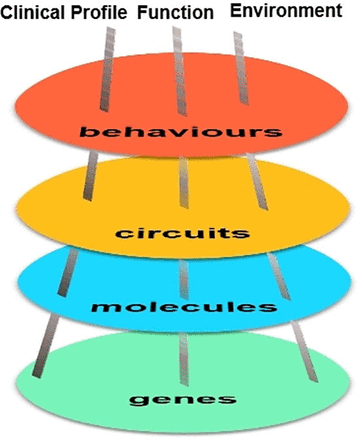
At present, there have been extensive cohort studies performed abroad; however, most of the world-leading cohorts are birth cohort studies.5 15 There are a few cohort studies of adolescents with depression, with sample sizes ranging from 200 to 500 and follow-up periods of up to 5 years,16 17 but overall there is a lack of large-scale cohort studies on adolescents with depression, especially those with a multidimensional comprehensive framework design.18 There is no previous cohort study specifically of adolescents with depression in China. To address this gap, our team has embarked on a cohort study on the Symptomatic trajectory and Biomarkers of Early Adolescent Depression. Given the main deficiencies in the current process of searching for biomarkers of depression, we designed a comprehensive framework of multidomain and multilevel units (figure 1) and facilitated the identification of biomarkers reflecting relationships among genetic, molecular neural circuitry levels and behavioural level abnormalities, all pointing to the development of early interventions for adolescents with depression and reducing the burden of disease.
Comprehensive framework of multidomain and multilevel units in our cohorts. The multidomain in this cohort included clinical profile, functional outcome, environmental risk factors and biological information. Multilevel units in this cohort stands for different levels of biological information, including genes, molecules, circuits and behaviours.
Objective
Main objective
The main purpose of this comprehensive longitudinal framework study is to develop objective markers for the diagnosis, disease progression and response to treatment, guiding early identification and intervention for adolescent depression.
Secondary objectives
We are tracking the trajectory of clinical symptoms, diagnosis and functional outcomes over a prolonged follow-up period, providing detailed information about time-dependent heterogeneity of clinical symptoms in Chinese adolescents with depression.
Furthermore, we will use comprehensive longitudinal data to perform neurobiologically-based diagnostic classification, independent of disorder-based categories.
Methods
Study design and participants
This multidimensional cohort study of adolescents with depression began in July 2021 and will end in June 2026. It is being carried out at the Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, China.
Considering that the average time from the first depressive episode to mania in depressed patients is 2–4 years, and the conversion rate from depression to bipolar disorder is highest in the first 5 years,19 we planned a 5-year follow-up period. The first available face-to-face clinical interview was taken at the baseline time point (T1) for each participant. After that, participants were/will be followed up separately at T2 (week 4), T3 (week 8), T4 (week 12), T5 (week 24), T6 (year 1), T7 (year 2), T8 (year 3), T9 (year 4) and T10 (year 5). We planned to recruit more than 1000 adolescents with depression and 300 healthy controls from July 2021 to June 2026. Of note, we are using the Bipolar Prodrome Symptom Scale-Full Prospective (BPSS-FP) to assess bipolar high-risk syndrome for individuals who experience subthreshold manic symptoms.20 When a patient meets the bipolar high-risk syndrome threshold, they are enrolled in a bipolar disorder high-risk subcohort and are followed for possible progression into bipolar disorder. In addition to the regular follow-up time points mentioned above, this subcohort have unplanned visits during possible hypomanic episodes.
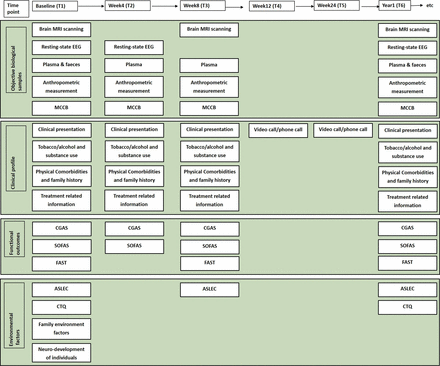
The study flowchart is presented in figure 2.
Flowchart of the multidimensional cohort study of adolescents with depression.
Participant eligibility criteria
The inclusion criteria for patients are as follows:
Meet the diagnostic criteria for MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).21
Drug-naive or continuous psychiatric pharmacotherapy for less than 14 days.
Aged 9–18 years.
Voluntary participation with written informed consent from the legal guardian.
The exclusion criteria for patients are as follows:
Neurological diseases, mental disorders due to brain diseases and physical diseases.
Developmental disorders, except for attention-deficit hyperactivity disorder, including IQ ≤70 or mental retardation, autistic spectrum disorder, obvious dyslexia and tic disorders.
Psychoactive substance use or abuse/dependence before depression onset.
Obvious active physical diseases.
The inclusion criteria for healthy controls are as follows:
No current or previous mental disorder according to DSM-5.
Aged 9–18 years.
Voluntary participation with written informed consent from the legal guardian.
The exclusion criteria for healthy controls are as follows:
Family history of mental disorders.
Neurological diseases.
Any kind of psychoactive substance use or self-injury.
Obvious active physical disease.
Data collection
Demographic information
Demographic information is collected by trained professionals using self-report tools at all time points, excluding T4, which is conducted through a video or telephone follow-up. Demographic information includes birth date, age, gender and education (including parents' educational information).
Clinical profile
We compiled a multidimensional clinical profile, including clinical presentation, tobacco/alcohol and substance use, physical health comorbidities, family history and treatment-related information. The constructs of these dimensions are shown in table 1.
Multidomains of clinical profile, constructs and assessments
Clinical presentation
Mental disorder diagnoses
Trained psychiatrists identified MDD (according to DSM-5) using the matched Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime (K-SADS-PL). Psychiatric comorbidities or diagnoses were also labelled as lifetime or current conditions at each time point.
Severity of symptom clusters
We focus on four common symptom clusters, including depressive, anxious, manic and psychotic symptoms. The severity of depressive symptoms is assessed with the 17-item Hamilton Depression Rating Scale (HAMD-17)22 and the Montgomery-Åsberg Depression Rating Scale (MADRS).23 The Hamilton Anxiety Scale (HAMA) is composed of 14 items with a total score of ≥7 indicating the presence of anxiety symptoms.24 The Young Mania Rating Scale is adopted to assess any hypomanic/manic symptoms.25 The six-item version of the Positive And Negative Syndrome Scale (PANSS-6), which has been shown to be sensitive to the effect of antipsychotic medication, is a measure of the severity of psychotic symptoms.26 In PANSS-6, there are three items each for the positive and negative subscale (P1 delusion, P2 conceptual disorganisation and P3 hallucinatory behaviour; N1 blunted affect, N4 passive/apathetic social withdrawal, and N6 lack of spontaneity and flow of conversation).
High-risk mental states assessment
Subthreshold clinical symptoms or high-risk symptoms have been previously indicated as risk factors for progression to more severe disorders.27 28 As such, to better characterise the full spectrum of depression in adolescents, we also focus on prodromal symptoms. In our cohort study, the subthreshold clinical symptoms include subthreshold depressive symptoms, subthreshold manic symptoms and psychotic high-risk states. According to prior studies, the subthreshold depressive symptoms are defined as (1) the participant experienced a minimum of three DSM-5 depressive symptoms, one of which included a depressed mood, but did not meet the full criteria for a major depressive episode, or the depression was otherwise not specified based on insufficient duration or severity; (2) symptoms represented a clear change from normal functioning, endorsed by self and others who know the person well, and (3) no evidence of major impairment.29 The subthreshold hypomanic symptoms are defined as (1) the participant experienced a minimum of three DSM-5 hypomanic symptoms but did not meet the full criteria for a hypomanic or manic episode or was otherwise not specified based on insufficient duration or severity; (2) symptoms represented a clear change from normal functioning, endorsed by self and others who know the person well, and (3) no evidence of major impairment. It is worth mentioning that we introduced the Chinese version of the BPSS-FP, a semi-structured assessment of the prodromal symptoms of bipolar disorder used in those adolescents with depression experiencing subclinical hypomanic symptoms.20 To our knowledge, this is the first study to introduce this instrument. Additionally, the Hypomania Checklist-32 (HCL-32) is used to self-assess hypomanic symptoms.27 Furthermore, the psychotic high-risk state also was surveyed as outlined in the Structured Interview for Prodromal Syndromes (SIPS).30
Sleep quality and circadian rhythm
The Pittsburgh Sleep Quality Index (PSQI) is a self-rating instrument to assess sleep quality over the previous 1 month, measuring the contents of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sedative-hypnotic drugs and daytime dysfunction.31 The Morningness-Eveningness Questionnaire (MEQ) characterises sleep–wake patterns and consists of 19 multiple choice items. According to the total score, the questionnaire categorises individuals into definite morning types and evening types.32
Self-injury and suicidal thoughts and behaviours
The Ottawa Self-Injury Inventory (OSI) is a self-report measure that assesses the functions and potential addictive features of non-suicidal self-injury (NSSI). We are adopting the Chinese version of OSI to measure the occurrence, frequency and the potential addictive features of self-injury.33
The Columbia Suicide Severity Rating Scale (C-SSRS) is a recognised scale that can quantify the severity of suicidal thoughts and suicidal behaviours, including the severity of suicidal thoughts, intensity of suicidal thoughts, suicidal behaviour subscale and lethality subscale.34
Tobacco/alcohol and substance use
Each time point in which tobacco, alcohol or any kind of psychoactive substance are recorded. Regarding tobacco use, we are adopting a smoking questionnaire focusing on current and previous smoking status. For individuals who smoke regularly, we are using the Fagerström Test for Nicotine Dependence (FTND) to assess tobacco dependence.35 A self-designed alcohol use and substance use questionnaire is adopted for their current and lifetime alcohol and substance use.
Physical health comorbidities and family history
Any previous and current physical illness of patients is recorded, as well as a family history that includes three diseases: hypertension, diabetes and thyroid disease;36this information is prepared for further genetic and epigenetic analysis. In addition, a family history of mental illnesses in first-, second- and third-degree relatives was ascertained by the Family Interview for Genetic Studies (FIGS).37
Treatment-related information
Information about patient treatment is recorded, including specific drugs, type of treatment, duration, number of hospitalizations, psychotherapy and physical therapy. In addition, information on the side effects of antidepressants and adherence to medication are also investigated. Treatment response has been performed for individuals who were first treated with the antidepressant fluoxetine or escitalopram within 16 weeks by the continuous change of the HAMD-17. A reduction rate of HAMD-17 above 50% from baseline to 8 weeks (T3) is defined as a response, while HAMD-17 total score ≤7 is defined as remission. The Clinical Global Impressions-Severity of Illness (CGI-S) ≤2 is also defined as remission.
Social and occupational functioning
A functional assessment using the Children’s Global Assessment Scale (CGAS) is assessed at each time point. To avoid confounding the rating with clinical symptoms, we adopted the Social and Occupational Functioning Assessment Scale (SOFAS) for functional assessment.29 Moreover, the Functioning Assessment Short Test (FAST) is used for self-reported occupational functioning.38
Environmental risk factors
The original Adolescent Self Rating Life Events Check (ASLEC) List includes 27 life events occurring over the last 6 months.39 With the purpose of quantifying persistent negative stress, we have expanded the occurrence of life events to the previous year. The Childhood Trauma Questionnaire (CTQ) is a recognised assessment to investigate different forms of childhood trauma before 16 years of age.40 Additionally, we are surveying family environmental factors, such as the number of family members, family income, marital status of parents, including divorce or separation. Additionally, individual risk factors for depression are evaluated, such as neurodevelopmental factors during the mother's pregnancy and perinatal period.
Objective measures
In our cohort, multilevel units of objective biological indicators are investigated, including neurocognitive function, plasma biomarkers, intestinal flora, anthropometric measurements (eg, height and weight), resting-state electroencephalography (EEG), and structural and functional magnetic resonance imagings (MRIs). All biological sample collections are performed after informed consent and within 3 days of clinical evaluation. Detailed assessment tools and metrics are summarised in table 2.
Standard assessments and detailed assessments of objective measures
Neurocognitive function
In our cohort study, neurocognitive function is characterised by using the Chinese version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB), which has demonstrated clinical validity and test–retest reliability in patients with MDD.41 Taking into account the uniqueness of the adolescent population and time constraints, we choose assessment of four domains of information processing speed, verbal learning, visual learning, and working memory, which consisting of six items of Trail Making Test Part A, Brief Assessment of Cognition in Schizophrenia: Symbol Coding (BACS-SC), Hopkins Verbal Learning Test (HVLT), Brief Visuospatial Memory Test-Revised (BVMT-R), Wechsler Memory Scale Spatial span and Category Fluency.
Biological sample collection
Blood samples are separated into plasma and red blood cells (for DNA isolation). Whole blood is also collected in Tempus tubes for direct isolation of RNA. Faeces from participants are partly collected for intestinal flora analysis. Brain electrical activity and brain MRI are also recorded.
Resting-state electroencephalography
The EEG data are collected through a 30-channel EEG cap (LT 37) following the extended 10–20 system, and the right mastoid is taken as a signal reference. The sampling rate is set to 250 Hz. We are adopting a 40-channel amplifier NuAmps (Compumedics; Neuroscan, Australia) to collect EEG signals for part of the participants. During data collection, all electrode impedances are kept below 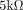 to ensure signal quality.
to ensure signal quality.
Brain structure and function
MRI scans are performed within 3 days of clinical assessment and biological sample collections. All adolescents are scanned for structural and functional MRI data on a 3.0 Tesla MRI system. Foam pads and headphones are used to minimise head motion and scanner noise. High-resolution T1-weighted images are collected using a fast gradient echo sequence with the following parameters: repetition time (TR)/echo time (TE)=8.2 ms/3.8 ms, field of view (FOV)=256×256 mm2, matrix=256×256, sagittal slices=188, thickness=1 mm, and voxel size=1×1×1 mm3. Functional MRI data are acquired using a gradient-echo echo-planar imaging sequence with the following parameters: TR=2000 ms, TE=30 ms, flip angle (FA)=90 degrees, FOV=220×220 mm2, matrix=64×64, axial slices=33, thickness=4 mm, gap=0.6 mm and voxel size=3.44×3.44×4 mm3.
Follow-up
The patient group is invited to complete all the follow-up assessments. We planned several ways to improve the follow-up rate. First, our team has sufficient human resources, including two full-time research psychiatrists, three research assistants, two graduate students and a specialist for project management. Both researchers and clinical psychiatrists fully collaborate, promoting a successful follow-up. Second, the division of labour is clear. A specialist is responsible for the follow-up contact and fixed, accountable assistants are responsible for the follow-up reception and scheduling of each of the examinationss, which guarantees good communication between our team and the adolescents' families. Third, we fully inform participants and their families to ensure they understand the programme's purpose and benefits at baseline enrolment; this could be further elaborated in follow-up sessions. Fourth, we provide practical help to patients (eg, offering ways for recording their mood changes throughout the day, training for early recognition of hypomania, assisting with doctors’ appointments when necessary, and giving psychological advice when needed). Fifth, we invite parents into an exclusive WeChat group after the enrolment and send them articles about mood disorders to improve the families' awareness of the disorders.
Specific follow-up times and the content of the multidimensional framework assessment are summarised in figure 3.
Multidimensional assessment and specific follow-up times. ASLEC, Adolescent Self Rating Life Events Check; CGAS, Children's Global Assessment Scale; CTQ, Childhood Trauma Questionnaire; EEG, electroencephalography; FAST, Functioning Assessment Short Test; MCCB, Measurement and Treatment Research to Improve Cognition In Schizophrenia (MATRICS) Consensus Cognitive Battery; MRI, magnetic resonance imaging; SOFAS, Social and Occupational Functioning Assessment Scale.
Statistical analysis
All data are collected by Epidata3.0. Data analysis will be done using the Statistical Package for Social Sciences 22 (SPSS 22). Statistical significance will be set at two-sided p<0.05 for behavioural and demographic data. Group differences in demographic, clinical, cognitive function data and cortical thickness will be assessed via analysis of variance or χ2 tests where relevant. The data of pretreatment/post-treatment will be analysed by the paired sample t-test. We will use multinomial models to analyse drug predictive associations with adolescent depression. For the trajectory of clinical symptoms analysis, according to previous studies, we will use a novel form of growth mixture modelling with structured residuals (GMM-SR) in MPlus (V.8.0) to classify youths into distinct subgroups.42
Strengths and limitations of this study
The current study addresses the lack of longitudinal cohort studies with large depression samples in Chinese adolescents. It will provide information on the trajectory of clinical symptoms in Chinese adolescents in the real world. Furthermore, it will track multidimensional outcomes, including circadian rhythm, physical comorbidity, tobacco/alcohol and substance use, self-injury and suicide, which can provide rich information to paediatric psychiatrists for early intervention. Most importantly, we launched a comprehensive framework with a long-term longitudinal study, collecting multiple units of objective markers at different time points. This framework fully considers the complex clinical heterogeneity of adolescent depression, facilitating the identification of biomarkers reflecting relationships among genetic, molecular neural circuitry levels and behavioural-level abnormalities. Our study will inform the development of early interventions in adolescents with depression and reduce the burden of the disease. The disadvantage of this comprehensive study is that acquisition of clinical information will occur over a long period, which may lead to an increased rate of subject loss. We will take measures, such as step-by-step evaluation and strengthening health education, to encourage patient follow-up.
Data availability statement
There are no data in this work.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and approval was obtained from the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University. Approval ID is (2021) No. (045). Written informed consent was obtained from adolescents and caregivers before collecting data.
Acknowledgments
Special thanks to Professor Zhijun Zhang of the Shenzhen Institute of Advanced Technology and Professor Jing Liu of Peking University Sixth Hospital for their guidance on clinical design.
References
Dr Xiaofei Zhang obtained a bachelor's degree of clinical medicine in 2012 and a master's degree in Psychiatry and Mental Health from Guangzhou Medical University, Guangzhou, China in 2015. She has been working at the Affiliated Brain Hospital of Guangzhou Medical University in China since 2015, where she is currently an attending psychiatrist. Her research interests include the genetic and brain imaging mechanisms of depression.

Footnotes
Contributors LC was responsible for this study. LC, XZ, YZ, XZ and CY conceived and designed the study. XZ, YZ, JC, ZL, JS, XC, RY, XC and LC participated in drafting the protocol and preparing the manuscript. XZ, YZ, JC, ZL, JS, XC, RY, XC, CY, XZ and LC read and approved the final manuscript.
Funding This work was supported by the National Key R&D Program of China (grant number 2022ZD0211700); National Natural Science Foundation of China (grant no. 81771466); Health Science and Technology Project of Guangzhou (grant no. 20211A010037); Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory, Guangzhou, China (grant no. 201805010009).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.