Article Text
Abstract
Background Impaired sensitivity of the skin flush response to niacin is one of the most replicated findings in patients with schizophrenia. However, prior studies have usually focused on postonset psychosis, and little is known about the clinical high-risk (CHR) phase of niacin sensitivity in psychosis.
Aims To profile and compare the niacin flush response among CHR individuals (converters and non-converters), patients with first-episode schizophrenia (FES) and healthy controls (HCs).
Methods Sensitivity to four concentrations (0.1–0.0001 M) of aqueous methylnicotinate was tested in 105 CHR individuals, 57 patients with FES and 52 HCs. CHR individuals were further grouped as converters and non-converters according to the 2-year follow-up outcomes. Skin flush response scores were rated on a 4-point scale.
Results Of the 105 CHR individuals, 21 individuals were lost during the study, leaving 84 CHR individuals; 16 (19.0%) converted to full psychosis at 2 years of follow-up. Flush response scores identified in the CHR samples were characterised as modest degree levels, intermediate between those of HC individuals and patients with FES. The flush responses in the CHR group mimicked the responses observed in the FES group at higher concentrations (0.01 M, 0.1 M) and longer time points (15 min, 20 min); however, these became comparable with the responses in the HC group at the shorter time points and at lower concentrations. The converters exhibited lower mean flush response scores than the non-converters.
Conclusions Attenuated niacin-induced flushing emerged during the early phase of psychosis. New devices should be developed and verified for objective quantification of skin responses in the CHR population.
- schizophrenia
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known on this topic
Subsensitivity of the skin flush response to niacin has been widely reported in patients with schizophrenia. This supports the notion that the subsensitivity to niacin is an endophenotype of schizophrenia. It is important to determine whether the subsensitivity to niacin is also common in individuals with clinical high risk (CHR) of psychosis.
What this study adds
The current study investigated niacin sensitivity in a relatively large number of drug-naive patients with first-episode schizophrenia (FES), individuals with CHR, and healthy controls (HCs). Compared with the FES and HC groups, our data showed that subsensitivity to niacin identified in the CHR samples was characterised by a modest level of severity that is intermediate between HC individuals and patients with FES.
How this study might affect research, practice or policy
The current study provides evidence that blunted flush responses to niacin appear in the early stage of psychosis and are associated with the disease progression, calling for implementation of more specific strategies (eg, supplementation of eicosapentaenoic acid) for early prevention or treatment.
Introduction
While there is obvious heterogeneity in the clinical manifestations and mechanisms in patients with psychosis,1 biological markers for the early identification of psychosis remain undiscovered.2 In particular, trait markers with endophenotypic characteristics3 4 that can be applied in the premorbid phase to increase power by predicting the outcome of conversion to psychosis are desirable. Blunted skin flush response to niacin has been widely reported in patients with psychosis5–7 and nonpsychotic first-degree relatives.8 9 Attenuated niacin response in patients with psychosis has been consistently reported in previous studies,6 10 and such abnormalities are heritable traits within psychosis-affected families.11 Other indirect evidence has shown that diminished flush responses are stable across time and depend on the stage of illness.12 These findings imply that the attenuated niacin flush response may be a useful trait for the early identification of psychosis.
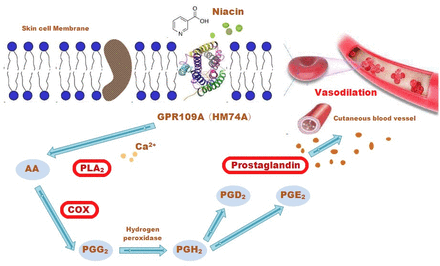
Although there are many hypotheses regarding the pathogenic mechanism of biochemical metabolism-related factors in psychosis,13 the mechanism of the attenuated skin flush response to niacin has been studied in-depth and found to be associated with disordered signalling in the phospholipase A2 (PLA2)-arachidonic acid (AA)-prostaglandin cascade.14 Niacin interacts with a specific G-protein-coupled receptor,15 leading to the active release of AA, which is mediated by PLA2 located at cell membranes. AA interacts with cyclo-oxygenase (COX), leading to its conversion to vasodilatory prostaglandins6 16 (figure 1). Thus, there is a subgroup of patients with psychosis characterised by an attenuated niacin flush response linked to the disordered GPR109A-COX-prostaglandin pathway,17 which can be used as a biomarker for predicting a subtype of psychosis from the premorbid phase.
The mechanism of niacin-induced skin flushing. AA, arachidonic acid; COX, cyclo-oxygenase; Ca2+, calcium; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGG2, prostaglandin G2; PGH2, prostaglandin H2; PLA2, phospholipase A2.
With rapid progress being made around the world in the identification of individuals at clinical high risk (CHR) for psychosis, there is now hope that psychosis can be treated to postpone and prevent the conversion to a first psychotic episode. Among individuals with CHR, about 20% are at risk of conversion within 2 years;18–20 efforts are currently underway to refine risk identification strategies to increase their predictive power. While there have been promising results from blunted niacin-induced skin flush responses in psychosis,21 whether this biomarker has early warning value in the prodromal phase of psychosis, such as in the CHR population, remains unknown. In 2017, Langbein et al22 administered an optical reflection spectroscopy method to test the sensitivity to three concentrations (0.1–0.001 M) of aqueous methylnicotinate (AMN) in 84 participants with CHR and 105 patients with first-episode psychosis. They found blunted niacin-induced skin flush reactions in the CHR individuals, similar to the previously reported impairment of the niacin response in psychosis. In contrast, in 2016, Berger et al23 compared the visual ratings of niacin sensitivity between CHR adolescents and healthy controls (HCs) and first-episode schizophrenia (FES) subjects. They found that the niacin sensitivity of the entire CHR group was significantly increased compared with the HC group, whereas there was no difference between converters and non-converters after 1-year follow-up.
In this study, we employed a larger CHR cohort (105 CHR individuals with a 2-year follow-up) from the Shanghai At-Risk for Psychosis-extended Program (SHARP-extended), a disease control population with FES, and an HC population. We used a 4-point scale measurement to compare the niacin flush responses in different stages of psychosis, with the aim of identifying the biomarker properties of niacin flush responses, namely, the differential response in the FES, CHR and HC groups. The identification of such CHR subgroups will hopefully lead to the development of a method for the precise prediction and personalised prevention of psychosis in the future.
Methods
Sample and procedures
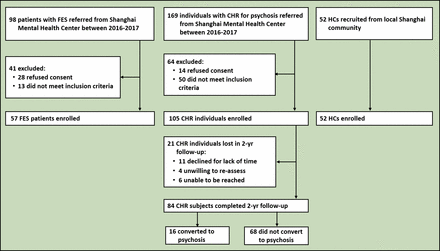
This was an observational study of 105 individuals who were confirmed to have CHR through face-to-face interviews. A total of 57 patients with FES were included as the disease control group and 52 subjects were used as HCs. The CHR individuals were from the SHARP-extended cohort; their samples were recruited between 2016 and 2021 at the Shanghai Mental Health Center (SMHC)24 in China. All participants agreed to participate in the study. Subjects younger than 18 years of age had their consent forms signed by their parents, and the youths gave informed assent. The patients fulfilled at least one of the prodromal syndrome criteria: (1) Brief intermittent psychotic syndrome, (2) Attenuated positive symptom syndrome, or (3) Genetic risk and deterioration syndrome. The inclusion criteria were as follows: (1) Age younger than 45 years old; (2) Individuals younger than 18 years of age had to be accompanied by either a parent or legal guardian; (3) Capacity to provide informed consent or assent if under 18 years; (4) Must have completed at least 6 years of primary education; and (5) Psychotropically naïve. The exclusion criteria were: (1) Severe somatic diseases, such as pneumonia, cancer or heart failure; (2) Intellectual disability; or (3) History of drug (such as methamphetamine) abuse or dependence. Zhang et al25–27 provided further details regarding the SHARP methodology.
The research procedure was independent of the routine clinical treatment procedures at the SMHC. In the present study, of the 105 CHR individuals who completed the baseline assessment, 21 individuals were lost, and 84 individuals completed both the baseline assessment and the 2-year follow-up (figure 2). Recruited CHR individuals were followed up every 6 months until the end of 24 months and reassessed by telephone or face-to-face interview every 6 months using the Structured Interview for Prodromal Syndromes (SIPS).28 29 The patients with FES met the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) diagnostic criteria for schizophrenia through the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) interview and had their first psychotic episode in the past year. Fifty-two HC individuals with a negative family history of mental disorders were recruited from the local community in Shanghai and matched to the FES group according to age, sex and education.
Flowchart showing study subjects selection. CHR, clinical high risk; FES, first-episode schizophrenia; HC, healthy controls.
Clinical measurement and outcome criteria
The SIPS was used to identify individuals with CHR. It consists of 19 items that assess four symptom domains: positive symptoms (scales P1–P5: P1 unusual thought content; P2 suspiciousness; P3 grandiosity; P4 perceptual abnormalities; and P5 disorganised communication), negative symptoms (scales N1–N6: N1 social anhedonia; N2 avolition; N3 expression of emotion; N4 experience of emotions and self; N5 ideational richness; and N6 occupational functioning), disorganised symptoms (scales D1–D4: D1 odd behaviour or appearance; D2 bizarre thinking; D3 trouble with focus and attention; and D4 impaired personal hygiene), and general symptoms (scales G1–G4: G1 sleep disturbance; G2 dysphoric mood; G3 motor disturbances; and G4 impaired tolerance to normal stress). During the SIPS interview, the global assessment of function (GAF) was used to measure the participants’ global psychological, social, and occupational functioning. Decreases in GAF scores were used to assess functional deterioration (ie,scores compared with the scores 12 months prior) during the SIPS interview.
Conversion to psychosis was the major outcome of this study. Of the remaining 84 CHR individuals, 16 (19.0%) converted to full psychosis at 2 years of follow-up. Conversion to psychosis was defined using the presence of psychotic symptoms in the SIPS29 30 criteria. The conversion was defined as the development of at least one psychotic-level symptom (with a rating of ‘6’ on the SIPS positive symptoms scale) with either sufficient frequency or duration, or occurring at least an hour a day on average over 4 days a week for at least 16 hours.
Measurement of the niacin response
Before the niacin skin patch test, participants were screened for the following criteria: no major systemic illness (especially heart disease, allergic skin illnesses and asthma), and no use of anti-inflammatory drugs (eg, aspirin, non-steroidal anti-inflammatory drugs and steroids) within 7 days before the test. The temperature may affect the skin’s response to niacin; therefore, the temperature in the test room was maintained at 25 ℃. A round filter paper patch was used to apply niacin in the form of AMN. Different concentrations (0.1 M, 0.01 M, 0.001 M and 0.0001 M) of AMN solution were prepared on the same day of the test. To better set the reference distance, a sticky ruler was attached to the inner side of the participant’s forearm. Four wet paper patches from each of the four AMN solutions were applied to four neighbouring sites on the forearm skin for 1 min and then removed. The skin flush response was photographed from a fixed vertical view at 5 min, 10 min, 15 min and 20 min after patch removal.7 The skin flush response was rated as follows: 0, no erythema; 1, incomplete erythema; 2, complete erythema within the defined area of the patch; and 3, erythema plus oedema beyond the definite area of the patch.31 The scoring of skin flush response was performed first by a research assistant and then by a senior researcher. Any inconsistent scores were discussed and evaluated together. Both groups were blinded to the grouping information. The niacin-induced flushing response scores consisted of 16 score values obtained at different AMN concentrations (0.0001 M, 0.001 M, 0.01 M, 0.1 M) and at different time points (5 min, 10 min, 15 min and 20 min).
Statistical analysis
Demographic and baseline clinical features are presented separately. Quantitative variables are expressed as mean (SD), while qualitative variables are presented as frequencies (%). The two groups were compared using χ2 tests for comparisons of categorical variables, rank-sum tests for comparisons of individual SIPS item scores and independent t-tests for comparisons of continuous variables. The dependent variables included the 16 raw scores obtained at different AMN concentrations (0.0001 M, 0.001 M, 0.01 M and 0.1 M) and at different time points (5 min, 10 min, 15 min and 20 min). CHR individuals were further grouped as converters and non-converters according to the 2-year follow-up outcomes. Multivariate analysis of variance (MANOVA) was conducted with age, education and group as the independent variables and the 16 raw scores in the skin flush response as the dependent variables for group comparisons and to assess covariate effects. Considering gender imbalance, the flush scores were compared using the MANOVA test for converters and non-converters. The level of statistical significance was set at a two-tailed p value of 0.05.
Results
Sample characteristics
Sex, height and weight were not significantly different among the FES, CHR and HC groups. The FES and HC groups were much older than the CHR group. The mean SIPS scores for the CHR and Positive and Negative Syndrome Scale (PANSS) measures for the FES group are listed in table 1.
Demographic and clinical variables, comparison among FES, CHR and HC groups
Comparison of the niacin response in the FES, CHR and HC groups
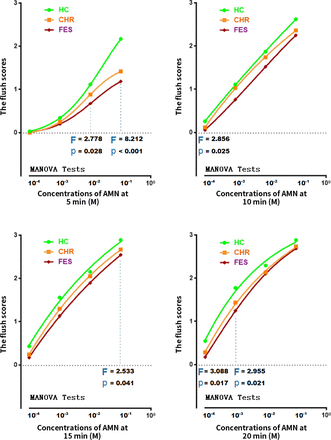
The MANOVA showed that the covariates of age and education had no impact on our effort to compare 16 raw scores in the skin flush response among three groups. The mean (SD) values of the niacin response for the FES, CHR and HC groups are shown in figure 3. Overall, many scores in the flush response were significantly different among the groups. The patients with FES exhibited lower mean flush response scores than the HC and CHR groups. Flush response scores identified in the CHR samples were characterised by a modest degree level, intermediate between those of HC individuals and patients with FES. The flush responses in the CHR group mimicked the responses observed in the FES group at the higher concentrations (0.01 M, 0.1 M) and longer time periods (15 min, 20 min); however, these became comparable with the responses in the HC group in the shorter time periods and lower concentrations.
A dot and line figure of means of the flush scores in niacin skin tests at four concentrations of AMN and at each time point in HCs, subjects with CHR and patients with FES. Note: Differences in the flush scores in niacin skin were presented. P values were calculated by MANOVA with age and education as covariates. AMN, aqueous methylnicotinate; CHR, clinical high risk; FES, first-episode schizophrenia; HCs, healthy controls; MANOVA, multivariate analysis of variance.
Table 2 highlights the significant differences between converters and non-converters in the baseline demographic variables. Male subjects, individuals with lower baseline or current GAF scores, and individuals with higher total SIPS scores were more likely to convert to psychosis during the follow-up. Comparisons of the baseline demographic, clinical and niacin-induced flushing response variables between those who quit and those who completed follow-up can be found in the online supplemental table S1. No significant differences in demographic and clinical characteristics between these two groups were found. However, significant differences were observed for the niacin-induced flushing response between the two groups. The CHR individuals in the former showed lower mean flush response than those subjects who completed the follow-up.
Supplemental material
Baseline demographic and SIPS variables, comparison between converters and non-converters.
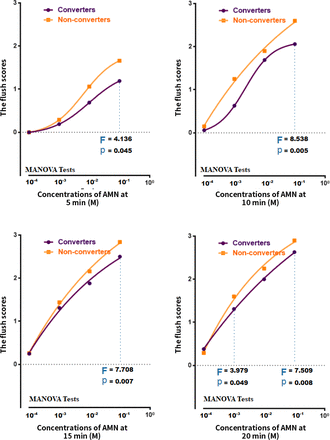
The mean scores of the flush responses for converters and non-converters are shown in figure 4. Overall, the converters exhibited lower mean flush response scores than the non-converters. The flush responses in the converter group differed from those observed in the non-converter group at the highest concentration (0.1 M); however, they became comparable at shorter time points and at lower concentrations.
A dot and line figure of means of the flush scores in niacin skin tests at four concentrations of AMN and at each time point in converters and non-converters for subjects with CHR. Note: Differences between converters and non-converters in the flush scores in niacin skin were presented. P values were calculated by the MANOVA test. AMN, aqueous methylnicotinate; CHR, clinical high risk; MANOVA, multivariate analysis of variance.
Discussion
Main findings
The first aim of this study was to investigate niacin sensitivity in CHR individuals compared with patients with FES, who met the criteria for schizophrenia for the first time, and with HCs. We found that niacin sensitivity of our CHR sample was attenuated, but to a lesser extent than in FES subjects. At higher concentrations or longer time points, the flush responses in the CHR group were close to the responses observed in the FES group, while at shorter time points or at lower concentrations, the flush responses became comparable with the responses in the HC group. Second, we investigated whether baseline niacin sensitivity differed between those who progressed towards psychosis (converters) versus those who did not (non-converters). We found that niacin sensitivity was more attenuated in the CHR converters than in the non-converters, especially at the highest concentration. To the best of our knowledge, this is the first attempt to investigate the niacin-induced flush response in a relatively large-scale CHR cohort in a Chinese clinical setting.
Compared with the FES and HC groups, our data showed that attenuated flush responses were identified in the CHR samples; these were characterised by a modest level of severity that was intermediate between HC individuals and patients with FES. Specifically, at a shorter time point (10 min), the flush responses in the CHR group were close to those in the HC group, while at longer durations and greater concentrations, differences between the CHR and FES groups were not observed, which implied that attenuated flush responses might already be present prior to the onset of full psychosis. Compared with HCs, patients with FES showed blunted flush responses, which was consistent with previous studies.3 6 32 Furthermore, the results in the CHR group in the current study replicate previous findings by Langbein et al in a different CHR sample.22 A novel finding is that niacin-induced flush responses appear to be attenuated in the CHR group, with similar alterations to the HC group at the lower concentrations and shorter time points but similar alterations to the FES group at higher concentrations and longer time points. This could indicate that the application of the niacin test in risk groups needs to be tailored to improve the accuracy of identification.
In the context of CHR follow-up research, the association of a biological marker such as attenuated flush responses and conversion outcomes is particularly valuable, as it helps to assign a specific pathological mechanism (and its extent) to refine risk identification strategies to increase predictive power for the CHR population. We identified several flush response scores that were significantly different between converters and non-converters, with converters showing a lower response to niacin than non-converters and more similar responses to those in patients with FES. Although the sample size of converters was small, the distinctiveness of their baseline niacin-induced responses was established, which is highly valuable in clinical practice for predicting psychosis since only 20% of CHR individuals would convert to psychosis in the near future. The results of the differences between converters and non-converters were similar to previous CHR studies;22 however, they were inconsistent with the recent findings by Berger et al that failed to find a difference between converters and non-converters.23 The heterogeneity of CHR samples may lead to inconsistent results regarding the degree of sensitivity to niacin. Langbein et al22 suggested the necessity for investigating biological markers in each subgroup of individuals who are at risk, defined by psychometric measures and their family history. The assumed properties of niacin responses in patients with psychosis may be that attenuated flush responses are correlated with negative symptom scores10 but inversely correlated with positive symptom scores.22
Although the visual method of measuring the niacin-induced flush responses in this study found group differences to a certain extent, the 4-point scale is subjective and unreliable; thus, this method is far from being quantifiable and useful for accurate clinical applications in early psychosis. The laser Doppler flowmeter for the niacin test was introduced by Messamore in 2003; it measures microcirculation in a non-invasive way, which is a useful method to quantify the extent of skin flush responses. The cutaneous blood flow response to increasing concentrations of topical methylnicotinate was measured to derive the log10 (EC50) and maximal blood flow values from the dose-response curve of each subject.14 Another method, denoted optical reflection spectroscopy, introduced by Smesny et al,33 enables the objective assessment of colour changes and can be used to quantify skin responses. Recently, by applying artificial intelligence technologies for image recognition, our team developed a niacin test device with easy access and high credibility for quantifying skin responses. Details of this method can be found in a companion article by Chen et al.
Limitations
This study has several limitations. First, clinical symptoms were not assessed in the HC subjects, whereas the PANSS was applied to the FES group, and the SIPS was applied to the CHR group. Therefore, whether relevant variables from these measures could be confounders when analysing niacin responses remains unknown. Second, the niacin tests were performed only once. The evidence would have been strengthened if we had tested them multiple times. Third, the sample size of the converter group was small; therefore, the present findings from comparisons between the converter and non-converter groups are insufficient and require further study. Finally, the SHARP-extended CHR cohort was surveyed naturally. There were 61 CHR individuals treated with antipsychotics for at least 2 weeks during the follow-up period. Therefore, it remains unknown whether antipsychotics can affect conversion outcomes.
Implications
The current study provides evidence that blunted flush responses to niacin appear in the early stage of psychosis and are associated with the disease progression. This could represent a psychosis subgroup with a similar underlying pathophysiology. The association of membrane polyunsaturated fatty acids (PUFAs) with psychosis,34 35 and with niacin sensitivity (especially in AA PUFAs)36 has been shown previously. Together with our findings, we hypothesise that there is indeed a subgroup of early psychosis with blunted flush response phenotypes and an underlying pathophysiology of abnormal fatty acid spectrum in neural cell membranes that hampers neuron activity and signal transduction. Therefore, these patients may benefit from the implementation of more specific strategies (eg, supplementation of eicosapentaenoic acid)37 38 for early prevention or treatment.
Conclusion
In summary, SHARP-extended CHR individuals showed attenuated niacin-induced flush responses characterised by a modest level of severity that was intermediate between those of HCs and patients with FES. The niacin flush response is more blunted in CHR converters than in non-converters. Future research should develop and validate new devices for the objective quantification of skin responses. Previous reviews have shown that an attenuated niacin response is associated with abnormalities in membrane fatty acid composition and activation of neuroinflammatory processes.32 39 There are potential implications for the rational, early and precise identification of and intervention for psychosis.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Research Ethics Committee of the SMHC (No. 2020-100).
Acknowledgments
For the purpose of commemorate, Prof. Jeffrey Yao passed away on 22 March, 2018. He was founder and core member of this study.
References
Ranpiao Gan is a medicine postgraduate at Shanghai Jiao Tong University School of Medicine. She graduated from Chongqing Medical University in 2018 with a bachelor's degree in medicine. Since 2019, she has been engaged in the assessment of clinical high-risk for psychosis and the detection of niacin at the Shanghai Mental Health Center. Her main interest is the early identification and intervention of clinical high-risk for psychosis.

Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
RG and YW contributed equally.
JW and TZ contributed equally.
Contributors TZ, JW and RG conceptualised the paper. GW, YH, LX, YW, XT and JZ oversaw data collection and project development. XL, HL and TC were responsible for the statistical analyses. TZ, JW and RG drafted the manuscript and provided data interpretation. TZ was responsible for the overall content as the guarantor. All authors participated in finalising the manuscript and agreed upon the final version of the manuscript.
Funding This study was supported by the National Natural Science Foundation of China (82171544, 81971251, 81671329, 82001406 and 81871050), Science and Technology Commission of Shanghai Municipality (19441907800, 16ZR1430500, 19ZR1445200, 19ZR1445100, 17411953100, 21S31903100, 2018SHZDZX01, 19410710800, 19411969100, 19411950800), Shanghai Clinical Research Center for Mental Health (19MC1911100) and The Clinical Research Center at Shanghai Mental Health Center (CRC2018ZD01, CRC2018ZD04), Project of the Key Discipline Construction, Shanghai 3-Year Public Health Action Plan (GWV-10.1-XK18), Clinical Research Center at Shanghai Jiao Tong University School of Medicine (DLY201817, 20190102), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01, 2018SHZDZX05) and ZJLab. Foundation of Shanghai Mental Health Center(2020-FX-02).
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.