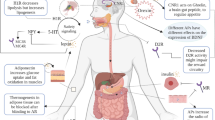
Abstract
Prescriptions for second-generation antipsychotics (SGAs) have surpassed those for first-generation agents in the treatment of schizophrenia and bipolar disorder. While SGAs have the benefit of a much reduced risk of causing movement disorders, they have been associated with weight gain and metabolic effects. These adverse reactions are not uncommon, and threaten to have a significant impact on the patient’s health over the long-term treatment that the patient requires. Currently, the aetiology of these effects is not known. This article reviews the data exploring the weight gain phenomenon. The literature was reviewed from searches of PubMed and the references of major articles in the field. The SGAs present a heterogeneous risk for weight gain. In addition, different individuals receiving the same drug can exhibit substantially different weight changes. This pattern suggests that a group of factors are associated with the weight gain phenomenon rather than a single mechanism. Coupled with the genetic profile that the patient brings to the treatment, the risk for
SGA-induced weight gain will be different for different drugs and different individuals. Targets for exploration of the weight gain phenomenon include receptor interactions involving serotonin, histamine, dopamine, adrenergic, cannabinoid and muscarinic receptors. The association of SGA-induced weight gain and the role of orexigenic and anorexigenic peptides are reviewed. Also, a brief discussion of genetic factors associated with SGA-induced weight gain is presented, including that of the serotonin 5-HT2C receptor gene (HTR2C) and the cannabinoid 1 receptor gene (CNR1).
The most promising data associated with SGA-induced weight gain include investigations of the histamine HP1, 5-HT2A, 5-HT2C, muscarinic M3 and adrenergic receptors. In addition, work in the genetic area promises to result in a better understanding of the variation in risk associated with different individuals.




Similar content being viewed by others
References
Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg 2003; 13: 329–30
Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–41
World Health Organization. World health report 2005 [online]. Available from URL: http://www.who.int/mediacentre/news/releases/2005/pr44/en/ [Accessed 2010 Jun 15]
Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA 2003; 289: 187–93
Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). Epub 2009 Jul 27
Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–23
Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009; 36: 341–50
Thakore JH. Metabolic disturbance in first-episode schizophrenia. Br J Psychiatry 2004; 184 Suppl. 47: S76–9
Spelman LM, Walsh PIN, Sharifi N, et al. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia. Diabet Med 2007; 24: 481–5
Venkatasubramanian G, Chittiprol S, Neelakantachar N, et al. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am J Psychiatry 2007; 164: 1557–60
Verma SK, Subramaniam M, Liew A, et al. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry 2009; 70: 997–1000
Sengupta S, Parrilla-Escobar MA, Klink R, et al. Are metabolic indices different between drug-naïve first-episode psychosis patients and healthy controls? Schizophr Res 2008; 102: 329–36
Padmavati R, McCreadie RG, Tirupati S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr Res 2010; 121: 199–202
McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005; 80: 19–32
Bobes J, Arango C, Aranda P, et al. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS study. Schizophr Res 2007; 90: 162–73
Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial: clinical comparison of sub-groups with and without the metabolic syndrome. Schizophr Res 2005; 80: 9–18
Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry 2004; 49: 753–60
Sugawara N, Yasui-Furukori N, Sato Y, et al. Prevalence of metabolic syndrome among patients with schizophrenia in Japan. Schizophr Res 2010; 123: 244–50
Baptista T, Serrano A, Uzcátegui E, et al. The metabolic syndrome and its constituting variables in atypical anti-psychotic-treated subjects: comparison with other drug treatments, drug-free psychiatric patients, first-degree relatives and the general population in Venezuela. Schizophr Res 2011; 126: 93–102
Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156: 1686–96
Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123: 225–33
Arif SA, Mitchell MM. Iloperidone: a new drug for the treatment of schizophrenia. Am J Health Syst Pharm 2011; 68: 301–8
Lee SY, Park MH, Patkar AA, et al. A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuro-Psychopharmacol Biol Psychiatry 2011; 35: 490–6
McIntyre RS. Asenapine: a review of acute and extension phase data in bipolar disorder. CNS Neurosci Ther. Epub 2010 Oct 15
Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Pharmacol 2008; 28(2 Suppl. 1): S20–8
Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 2009; 70: 829–36
Bai YM, Chen JY, Chen TT, et al. Weight gain with clozapine: 8-year cohort naturalistic study among hospitalized Chinese schizophrenia patients. Schizophr Res 2009; 108: 122–6
Basson BR, Kinon BJ, Taylor CC, et al. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine haloperidol, or risperidone. J Clin Psychiatry 2001; 62: 231–8
Boden R, Haenni A, Lindstrom L, et al. Biochemical risk factors for development of obesity in first-episode schizophrenia. Schizophr Res 2009; 115: 141–5
Saddichha S, Ameen S, Akhtar S. Predictors of anti-psychotic-induced weight gain in first-episode psychosis: conclusions from a randomized, double-blind, controlled prospective study of olanzapine, risperidone, and haloperidol. J Clin Psychopharmacol 2008; 28: 27–31
Bobes J, Rejas J, Garcia-Garcia M, et al. Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophr Res 2003; 62: 77–88
Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res 2009; 43: 620–6
Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry 2002; 63: 425–33
Safer DJ. A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 2004; 24: 429–36
Strassnig M, Miewald J, Keshavan M, et al. Weight gain in newly diagnosed first episode psychosis patients and healthy comparisons: one-year analysis. Schizophr Res 2007; 93: 90–8
Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res 2009; 110: 95–102
Kelly DL, Kreyenbuhl J, Love RC, et al. Six-month review of weight and metabolic parameters in patients receivingclozapine, risperidone, olanzapine, or quetiapine. J Clin Psychiatry 2003; 64: 1133–4
Newcomer JW, Ratner RE, Eriksson JW, et al. A 24-week, multicenter, open-label, randomized study to compare changes in glucose metabolism in patients with schizophrenia receiving treatment with olanzapine, quetiapine, or risperidone. J Clin Psychiatry 2009; 70: 487–99
Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medicationsduring first-time use in children and adolescents. JAMA 2009; 302: 1765–73
Fleischhaker C, Heiser P, Hennighausen K, et al. Clinical drug monitoring in child and adolescent psychiatry: side effects of atypical neuroleptics. J Child Adolesc Psychopharmacol 2006; 16: 308–16
Reynolds GP, Hill MJ, Kirk SL. The 5-HT2C receptor and antipsychotic induced weight gain-mechanisms and genetics. J Psychopharmacol 2006; 20: 15–8
Sikich L, Hamer RM, Bashford RA, et al. A pilot study of risperidone, olanzapine, and haloperidol in psychoticyouth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology 2004; 29: 133–45
Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000; 157: 975–81
Kinon BJ, Kaiser CJ, Ahmed S, et al. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol 2005; 25: 255–8
Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther 2010; 127: 210–51
Stahl SM, Meyer JM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand 2009; 119: 4–14
Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008; 13: 27–35
Perez-Iglesias R, Crespo-Facorro B, Amado JA, et al. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry 2007; 68: 1733–40
Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003; 28: 519–26
Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 2000; 68: 29–39
Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 2010; 334: 171–81
Woods SW, Martin A, Spector SG, et al. Effects of development on olanzapine-associated adverse events. J Am Acad Child Adolesc Psychiatry 2002; 41: 1439–46
Gothelf D, Falk B, Singer P, et al. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry 2002; 159: 1055–7
Kusumi I, Takahashi Y, Suzuki K, et al. Differential effects of subchronic treatments with atypical antipsychotic drugs on dopamine D2 and serotonin 5-HT2A receptors in the rat brain. J Neural Transm 2000; 107: 295–302
Huang X.-F, Han M, Huang X, et al. Olanzapine differentially affects 5-HT2A and 2C receptor mRNA expression in the rat brain. Behav Brain Res 2006; 171: 355–62
Sugimoto Y, Yoshikawa T, Yamada J. Effects of peripheral administration of 5-hydroxytryptamine (5-HT) on 2-deoxy-D-glucose-induced hyperphagia in rats. Biol Pharm Bull 2002; 25: 1364–6
Tulipano G, Rizzetti C, Bianchi I, et al. Clozapine induced alteration of glucose homeostasis in the rat: the contribution of hypothalamic-pituitary-adrenal axis activation. Neuroendocrinology 2007; 85: 61–70
Gilles M, Wilke A, Kopf D, et al. Antagonism of the serotonin (5-HT)-2 receptor and insulin sensitivity: implications for atypical antipsychotics. Psychosom Med 2005; 67: 748–51
Currie PJ, Coiro CD, Niyomchai T, et al. Hypothalamic paraventricular 5-hydroxytryptamine: receptor-specific inhibition of NPY-stimulated eating and energy metabolism. Pharmacol Biochem Behav 2002; 71: 709–16
Huang X-F, Deng C, Zavitsanou K. Neuropeptide Y mRNA expression levels following chronic olanzapine, clozapine and haloperidol administration in rats. Neuropeptides 2006; 40: 213–9
Huang XF, Tan YY, Huang X, et al. Effect of chronic treatment with clozapine and haloperidol on 5-HT(2A and 2C) receptor mRNA expression in the rat brain. Neurosci Res 2007; 59: 314–21
Wang Q, Huang XF. Effects of chronic treatment of olanzapine and haloperidol on peptide YY binding densities in the rat brain. Exp Neurol 2008; 209: 261–7
Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY (3-36) physiologically inhibits food intake. Nature 2002; 418: 650–4
Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 2003; 349: 941–8
Hajduch E, Rencurel F, Balendran A, et al. Serotonin(5-hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J Biol Chem 1999; 274: 13563–8
Vickers SP, Clifton PG, Dourish CT, et al. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology 1999; 143: 309–14
Hewitt KN, Lee MD, Dourish CT, et al. Serotonin 2C receptor agonists and the behavioral satiety sequence in mice. Pharmacol Biochem Behav 2002; 71: 691–700
Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 1995; 374: 542–6
Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 2000; 295: 226–32
Rauser L, Savage JE, Meltzer HY, et al. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor. J Pharmacol Exp Ther 2001; 299: 83–9
Nonogaki K, Strack AM, Dallman MF, et al. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med 1998; 4: 1152–6
Kirk SL, Neill JC, Jones DN, et al. Ziprasidone suppresses olanzapine-induced increases in ingestive behaviour in the rat. Eur J Pharmacol 2004; 505: 253–4
Snigdha S, Thumbi C, Reynolds GP, et al. Ziprasidone and aripiprazole attenuate olanzapine-induced hyperphagia in rats. J Psychopharmacol 2008; 22: 567–71
Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol 2009; 29: 165–9
Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003; 28: 1400–11
Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des 2010; 16: 488–501
Deng C, Chen J, Hu C, et al. What is the mechanism for aripiprazole’s effect on reducing olanzapine-associated obesity? J Clin Psychopharmacol 2010; 30: 480–1
Kirk SL, Glazebrook J, Grayson B, et al. Olanzapine induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology 2009; 207: 119–25
Heal DJ, Smith SL, Fisas A, et al. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther 2008; 117: 207–31
Caldirola P. 5-HT6 receptor antagonism: a novel mechanism for the management of diabetes. SMI Conference on Obesity and Related Disorders; 2003 Feb 17–18; London
Dupuis DS, Mannoury la Cour C, Chaput C, et al. Actions of novel agonists, antagonists and antipsychotic agents at recombinant rat 5-HT6 receptors: a comparative study of coupling to G alpha s. Eur J Pharmacol 2008; 588: 170–7
Wagstaff AF, Fitton A, Benfield P. Sulpiride: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in schizophrenia. CNS Drugs 1994; 2: 313–33
Baptista T, Hernandez L, Hoebel BG. Systemic sulpiride increases dopamine metabolites in the lateral hypothalamus. Pharmacol Biochem Behav 1990; 37: 227–9
Parada MA, Hernandez L, Hoebel BG. Sulpiride injections in the lateral hypothalamus induce feeding and drinking in rats. Pharmacol Biochem Behav 1988; 30: 917–23
Baptista T, LaCruz A, Meza T, et al. Antipsychotic drugs and obesity:is prolactin involved? Can J Psychiatry 2001; 46: 829–34
Correa N, Opler LA, Kay SR, et al. Amantadine in the treatment of neuroendocrine side effects of neuroleptics. J Clin Psychopharmacol 1987; 7: 91–5
Parada MA, Hernandez L, Paez X, et al. Mechanism of the sulpiride-induced obesity in rats. Pharmacol Biochem Behav 1989; 33: 45–50
Baptista T, Hertiandez L, Parada MA. Long term administration of some antipsychotic drugs increases body weight and feeding in rats:are dopamine D2 receptors involved? Pharmacol Biochem Behav 1987; 27: 399–405
Baptista T, de Baptista EA, Hernandez L et al. Tamoxifen prevents sulpiride-induced obesity in rats. Pharmacol Biochem Behav 1997; 57: 215–22
Baptista T, Lopez MA, Teneud L, et al. Amantadine in the treatment of neuroleptic-induced obesity in rats: behavioral, endocrine and neurochemical correlates. Pharmacopsychiatry 1997; 30: 43–54
Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002; 6: 601–9
Wang GJ, Volkow ND, Thanos PK, et al. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis 2004; 23: 39–53
Kaur G, Kulkarni SK. Studies on modulation of feeding behavior by atypical antipsychotics in female mice. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 277–85
Beaudry G, Zekki H, Rouillard C, et al. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse 2004; 51: 233–40
Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov 2008; 7: 41–53
Bouthenet ML, Ruat M, Sales N, et al. A detailed mapping of histamine H1-receptors in guinea pig central nervous system established by autoradiography with [125I] iodobolpyramine. Neuroscience 1988; 26: 553–600
Fukagawa K, Sakata T, Shiraishi T, et al. Neuronal histamine modulates feeding behavior through H1-receptor in rat hypothalamus. Am J Physiol 1989; 256: R605–11
Magrani J, de Castro e Silva E, Varjão B, et al. Histaminergic H1 and H2 receptors located within the ventromedial hypothalamus regulate food and water intake in rats. Pharmacol Biochem Behav 2004; 79: 189–98
Masaki T, Chilba S, Yasuda T, et al. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes 2004; 53: 2250–60
Navarro-Badenes J, Martinez-Mir I, Palop V, et al. Weightgain associated with cinnarizine. Ann Pharmacother 1992; 26: 928–30
Orthen-Gambill N. Antihistaminic drugs increase feeding,while histidine suppresses feeding in rats. Pharmacol Biochem Behav 1988; 31: 81–6
Varsano I, Volovitz B, Soferman R, et al. Multicenter study with ketotifen (Zaditen) oral drop solution in the treatment of wheezy children aged 6 months to 3 years. Pediatr Allergy Immunol 1993; 4: 45–50
Wihl J-A, Nuchel Petersen B, Nuchel Petersen L, et al. Effect of nonsedative H1-receptor antagonist astemizole in perennial allergic and nonallergic rhinitis. J Allergy Clin Immunol 1985; 75: 720–7
Han M, Deng C, Burne TH, et al. Short- and long-term effects of antipsychotic drug treatment on weight gain and H1 receptor expression. Psychoneuroendocrinology 2008; 33: 569–80
Bymaster FP, Nelson DL, DeLapp NW, et al. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and alpha 1-adrenergic receptors in vitro. Schizophr Res 1999; 37: 107–22
Kim SF, Huang AS, Snowman AM, et al. Antipsychotic drug-induced weight gain mediated by histamine H1 receptor linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA 2007; 104: 3456–9
Kahn BB, Alquier T, Carling D, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15–25
Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–74
Lecklin A, Etu-Seppälä P, Stark H, et al. Effects of intracerebroventricularly infused histamine and selective H1, H2 and H3 agonists on food and water intake and urine flow in Wistar rats. Brain Res 1998; 793: 279–88
Sakata T, Fukagawa K, Ookuma K, et al. Hypothalamic neuronal histamine modulates ad libitum feeding by rats. Brain Res 1990; 537: 303–6
Morimoto T, Yamamoto Y, Mobarakeh JI, et al. Involvement of the histaminergic system in leptin-induced suppression of food intake. Physiol Behav 1999; 67: 679–83
Masaki T, Yoshimatsu H, Chiba S, et al. Targeted disruption of histamine H1-receptor attenuates regulatory effects of leptin on feeding, adiposity, and UCP family in mice. Diabetes 2001; 50: 385–91
Lian J, Huang X-F, Pai N, et al. Reduce the olanzapine-induced body weight gain with histamine H1 receptor agonist betahistine in rats [abstract no. P-04.051]. XXVII CINP Congress; 2010 Jun 6–10; Hong Kong. Int J Neuropsychopharmacol 2010; 13 Suppl. 1: 99
Poyurovsky M, Pashinian A, Levi A, et al. The effect of betahistine, a histamine H1 receptor agonist/H3 antagonist, on olanzapine-induced weight gain in first-episode schizophrenia patients. Int Clin Psychopharmacol 2005; 20: 101–3
Roerig JL, Steffen KJ, Mitchell JE, et al. An exploration of the effect of modafinil on olanzapine associated weight gain in normal human subjects. Biol Psychiatry 2009; 65: 607–13
Lundquist I, Ericson LE. Beta-adrenergic insulin release and adrenergic innervation of mouse pancreatic islets. Cell Tissue Res 1978; 193: 73–85
Cavero I, Roach AG. The pharmacology of prazosin, a novel antihypertensive agent. Life Sci 1980; 27: 1525–40
Ahrén B, Lundquist I, Järhult J. Effects of alpha 1-, alpha 2- and beta-adrenoceptor blockers on insulin secretion in the rat. Acta Endocrinol (Copenh) 1984; 105: 78–82
John GW, Doxey JC, Walter DS, et al. The role of alpha- and beta-adrenoceptor subtypes in mediating the effects of catecholamines on fasting glucose and insulin concentrations in the rat. Br J Pharmacol 1990; 100: 699–704
Savoy YE, Ashton MA, Miller MW, et al. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr Bull 2010; 36: 410–8
Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 2004; 3: 353–9
Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996; 14: 87–96
Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology 1999; 141: 267–78
Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet 2005; 20: 368–78
Silvestre JS, Prous J. Research on adverse drug events: I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Meth Find Exp Clin Pharmacol 2005; 27: 289–304
Gautam D, Han SJ, Hamdan FF, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 2006; 3: 449–61
Johnson DE, Yamazaki H, Ward KM, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic induced diabetes and hyperglycemia. Diabetes 2005; 54: 1552–8
Sowell MO, Mukhopadhyay N, Cavazzoni P, et al. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab 2002; 87: 2918–23
Teff KL, Townsend RR. Prolonged mild hyperglycemia induces vagally mediated compensatory increase in C-peptide secretion in human. J Clin Endocrinol Metab 2004; 89: 5606–3
Sasaki N, Iwase M, Uchizono Y, et al. The atypical antipsychotic clozapine impairs insulin secretion by inhibiting glucose metabolism and distal steps in rat pancreatic islets. Diabetologia 2006; 49: 2930–8
Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol 2005; 16: 297–313
Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America — a randomized controlled trial. JAMA 2006; 295: 761–75
Ameri A, Wilhelm A, Simmet T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol 1999; 126: 1831–9
Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 2007; 18: 27–37
Woods SC. Role of the endocannabinoid system in regulating cardiovascular and metabolic risk factors. Am J Med 2007; 120(3 Suppl. 1): S19–25
Dean B, Sundram S, Bradbury R, et al. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience 2001; 103: 9–15
Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res 2006; 172: 556–60
Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE 2008; 3: e1797
Mato S, Aso E, Castro E, et al. CB 1 knockout mice display impaired functionality of 5-HT 1A and 5-HT 2A/C receptors. J Neurochem 2007; 103: 2111–20
Darmani NA, Janoyan JJ, Kumar N, et al. Behaviorally active doses of the CB 1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol Biochem Behav 2003; 75: 777–87
Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822–5
Secher A, Husum H, Holst B, et al. Risperidone treatment increases CB1 receptor binding in rat brain. Neuroendocrinology 2010; 91: 155–68
Weston-Green K, Huang XF, Han M, et al. The effects of antipsychotics on the density of cannabinoid receptors in the dorsal vagal complex of rats: implications for olanzapine-induced weight gain. Int J Neuropsychopharmacol 2008; 11: 827–35
Orr J, Davy B. Dietary influences on peripheral hormones regulating energy intake: potential applications for weight management. J Am Diet Assoc 2005; 105: 1115–24
Black MD, Stevens RJ, Rogacki N, et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacology (Berl) 2011; 215: 149–63
Liebig M, Gossel M, Pratt J, et al. Profiling of energy metabolism in olanzapine-induced weight gain in rats and its prevention by the CB1-antagonist AVE1625. Obesity 2010; 18: 1952–8
Schwartz MW, Woods SC, Porte Jr D, et al. Central nervous system control of food intake. Nature 2000; 404: 661–71
Obuchowicz E. Long-term treatment with chlorpromazine and haloperidol but not with sulpiride and clozapine markedly elevates neuropeptide Y-like immunoreactivity in the rat hypothalamus. Neuropeptides 1996; 30: 471–8
Gruber SH, Mathe AA. Effects of typical and atypical antipsychotics on neuropeptide Y in rat brain tissue and microdialysates from ventral striatum. J Neurosci Res 2000; 61: 458–63
Angelucci F, Aloe L, Gruber SH, et al. Chronic antipsychotic treatment selectively alters nerve growth factor and neuropeptide Y immunoreactivity and the distribution of choline acetyl transferase in rat brain regions. Int J Neuropsychopharmacol 2000; 3: 13–25
Kirk SL, Cahir M, Reynolds GP. Clozapine, but not haloperidol, increases neuropeptide Y neuronal expression in the rat hypothalamus. J Psychopharmacol 2006; 20: 577–9
Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth hormone releasing acylated peptide from stomach. Nature 1999; 402: 656–60
Bowers CY. A natural growth hormone releasing peptide begets natural ghrelin. J Endocrinol Metab 2001; 86: 1464–9
Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000; 141: 4325–8
Tschop M, Smiley DL, Heiman ML. Ghrelin inducesadiposity in rodents. Nature 2001; 407: 908–13
Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–8
Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337–45
Cummings DE, Purnell JQ, Frayo RS, et al. A pre-prandial rise in plasma ghrelin level suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–9
Tschop M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001; 24: RC19–21
Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992–5
Murashita M, Kusumi I, Hosoda H, et al. Acute administration of clozapine concurrently increases blood glucose and circulating plasma ghrelin levels in rats. Psychoneuroendocrinology 2007; 32: 777–84
Vidarsdottir S, Roelfsema F, Streefland T, et al. Short-term treatment with olanzapine does not modulate gut hormone secretion: olanzapine disintegrating versus standard tablets. Eur J Endocrinol 2010; 162: 75–83
Sentissi O, Grouselle D, Viala A, et al. Ghrelin and leptin levels in schizophrenic patients treated with antipsychotic monotherapy. J Clin Psychopharmacol 2009; 29: 304–6
Kim BJ, Sohn JW, Park CS, et al. Body weight and plasma levels of ghrelin and leptin during treatment with olanzapine. J Korean Med Sci 2008; 23: 685–90
Tanaka K, Morinobu S, Ichimura M, et al. Decreased levels of ghrelin, cortisol, and fasting blood sugar, but not n-octanoylated ghrelin, in Japanese schizophrenic inpatients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1527–32
Perez-Iglesias R, Vazquez-Barquero JL, Amado JA, et al. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J Clin Psychopharmacol 2008; 28: 289–95
Esen-Danaci A, Sarandöl A, Taneli F, et al. Effects of second generation antipsychotics on leptin and ghrelin. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1434–8
Roerig JL, Mitchell JE, Steffen KJ, et al. A comparison of the effects of olanzapine and risperidone versus placebo on ghrelin plasma levels. J Clin Psychopharmacol 2008; 28: 21–6
Popovic V, Doknic M, Maric N, et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology 2007; 85: 249–56
Hosojima H, Togo T, Odawara T, et al. Early effects of olanzapine on serum levels of ghrelin, adiponectin and leptin in patients with schizophrenia. J Psychopharmacol 2006; 20: 75–9
Himmerich H, Fulda S, Kunzel HE, et al. Ghrelin plasma levels during psychopharmacological treatment. Neuropsychobiology 2005; 52: 11–6
Palik E, Birkas KD, Faludi G, et al. Correlation of serum ghrelin levels with body mass index and carbohydrate metabolism in patients treated with atypical antipsychotics. Diabetes Res Clin Pract 2005; 68 Suppl. 1: S60–4
Murashita M, Kusumi I, Inoue T, et al. Olanzapine increases plasma ghrelin level in patients with schizophrenia. Psychoneuroendocrinology 2005; 30: 106–10
Sporn AL, Bobb AJ, Gogtay N, et al. Hormonal correlates of clozapine induced weight gain in psychotic children: an exploratory study. J Am Acad Child Adolesc Psychiatry 2005; 44: 925–33
Theisen FM, Gebhardt S, Bromel T, et al. A prospective study of serum ghrelin levels in patients treated with clozapine. J Neural Transm 2005; 112: 1411–6
Togo T, Hasegawa K, Miura S, et al. Serum ghrelin concentrations in patients receiving olanzapine or risperidone. Psychopharmacology (Berl) 2004; 172: 230–2
Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–32
Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev 2002; 60(10 Pt 2): S1–14
Klein S, Coppack SW, Mohamed-Ali V, et al. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes 1996; 45: 984–7
Chan JL, Bluher S, Yiannakouris N, et al. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 2002; 51: 2105–12
Brömel T, Blum WF, Ziegler A, et al. Serum leptin levels increase rapidly after initiation of clozapine therapy. Mol Psychiatry 1998; 3: 76–80
Melkersson K, Dahl ML. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs 2004; 64: 701–23
Sentissi O, Epelbaum J, Olié JP, et al. Leptin and ghrelin levels in patients with schizophrenia during different antipsychotics treatment: a review. Schizophr Bull 2008; 34: 1189–99
Jin H, Meyer JM, Mudaliar S, et al. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr Res 2008; 10: 70–85
Ebenbichler C, Laimer M, Kranebitter M, et al. The soluble leptin receptor in olanzapine-induced weight gain: results from a prospective study. Schizophr Res 2005; 75: 143–6
Haupt DW, Luber A, Maeda J, et al. Plasma leptin and adiposity during antipsychotic treatment of schizophrenia. Neuropsychopharmacology 2005; 30: 184–91
Herran A, Garcia-Unzueta MT, Amado JA, et al. Effects of long-term treatment with antipsychotics on serum leptin levels. Br J Psychiatry 2001; 179: 59–62
Baptista T, Dávila A, El Fakih Y, et al. Similar frequency of abnormal correlation between serum leptin levels and BMI before and after olanzapine treatment in schizophrenia. Int Clin Psychopharmacol 2007; 22: 205–11
Maayan LA, Vakhrusheva J. Risperidone associated weight, leptin, and anthropometric changes in children and adolescents with psychotic disorders in early treatment. Hum Psychopharmacol Clin Exp 2010; 25: 133–8
Marquina D, Peña R, Fernández E, et al. Abnormal correlation between serum leptin levels and body mass index may predict metabolic dysfunction irrespective of the psychopharmacological treatment. Int Clin Psychopharmacol 2011; 26: 169–72
Peña R, Marquina D, Serrano A, et al. Frequency of abnormal correlation between leptin and the body mass index during first and second generation antipsychotic drug treatment. Schizophr Res 2008; 106: 315–9
Newcomer JW, Haupt DW, Fucetola R, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002; 59: 337–45
Chiu CC, Chen CH, Chen BY, et al. The time-dependent change of insulin secretion in schizophrenic patients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 866–70
Henderson DC, Cagliero E, Copeland PM, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents. Arch Gen Psychiatry 2005; 62: 19–28
Tandon R, Belmaker RH, Gattaz WF, et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 2008; 100: 20–38
Kahn RS, Fleischhacker WW, Boter H, et al., EUFEST Study Group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomized clinical trial. Lancet 2008; 371: 1085–97
Lencz T, Malhotra AK. Pharmacogenetics of antipsychotic-induced side effects. Dialogues Clin Neurosci 2009; 11: 405–15
Adan RA, Vanderschuren LJ, la Fleur SE. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci 2008; 29: 208–17
Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 2002; 359: 2086–7
Miller DD, Ellingrod VL, Holman TL, et al. Clozapine induced weight gain associated with the 5HT2C receptor -759C/T polymorphism. Am J Med Genet 2005; 133: 97–100
Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160: 677–9
Ellingrod VL, Perry PJ, Ringold JC, et al. Weight gain associated with the -759C/T polymorphism of the 5HT2C receptor and olanzapine. Am J Med Genet B Neuropsychiatr Genet 2005; 34: 76–8
Lane HY, Liu YC, Huang CL, et al. Risperidone-related weight gain: genetic and nongenetic predictors. J Clin Psychopharmacol 2006; 26: 128–34
Templeman LA, Reynolds GP, Arranz B, et al. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics 2005; 15: 195–200
Ryu S, Cho EY, Park T. -759 C/T polymorphism of 5-HT2C receptor gene and early phase weight gain associated with antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 673–7
Reynolds GP, Templeman LA, Zhang ZJ. The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1021–8
Tsai SJ, Hong CJ, Yu YW, et al. 759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain [letter]. Lancet 2002; 360: 1790
Basile VS, Masellis M, De Luca V, et al. 759C/T genetic variation of 5HT(2C) receptor and clozapine-induced weight gain. Lancet 2002; 360: 1790–1
Theisen FM, Hinney A, Brömel T, et al. Lack of association between the -759C/T polymorphism of the 5-HT2C receptor gene and clozapine-induced weight gain among German schizophrenic individuals. Psychiatr Genet 2004; 14: 139–42
De Luca V, Müller DJ, Hwang R, et al. HTR2C haplotypes and antipsychotics-induced weight gain: X-linked multimarker analysis. Hum Psychopharmacol 2007; 22: 463–7
Monteleone P, Milano W, Petrella C, et al. Endocannabinoid Pro129Thr FAAH functional polymorphism but not 1359G/A CNR1 polymorphism is associated with antipsychotic-induced weight gain. J Clin Psychopharmacol 2010; 30: 441–5
Tiwari AK, Zai CC, Likhodi O, et al. A common 2 receptor 1 (CNR1) gene is associated with antipsychotic-induced weight gain in schizophrenia. Neuropsychopharmacology 2010; 35: 1315–24
Van der Lende T, Te Pas MF, Veerkamp RF, et al. Leptin gene polymorphisms and their phenotypic associations. Vitam Horm 2005; 71: 373–404
Hoffstedt J, Eriksson P, Mottagui-Tabar S, et al. A polymorphism in the leptin promoter region (-2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res 2002; 34: 355–9
Zhang ZJ, Yao ZJ, Mou XD, et al. Association of -2548G/A functional polymorphism in the promoter region of leptin gene with antipsychotic agent-induced weight gain. Zhonghua Yi Xue Za Zhi 2003; 83: 2119–23
Ellingrod VL, Bishop JR, Moline J, et al. Leptin and leptin receptor gene polymorphisms and increases in body mass index (BMI) from olanzapine treatment in persons with schizophrenia. Psychopharmacol Bull 2007; 40: 57–62
Gregoor JG, van der Weide J, Mulder H, et al. Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J Clin Psychopharmacol 2009; 29: 21–5
Calarge CA, Ellingrod VL, Zimmerman B, et al. Leptin gene -2548G/A variants predict risperidone-associated weight gain in children and adolescents. Psychiatr Genet 2009; 19: 320–7
Ujike H, Nomura A, Morita Y, et al. Multiple genetic factors in olanzapine-induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry 2008; 69: 1416–22
Fujisawa T, Ikegami H, Kawaguchi Y, et al. Meta-analysis of the association of Trp64Arg polymorphism of beta3-adrenergic receptor gene with body mass index. J Clin Endocrinol Metab 1998; 83: 2441–4
Thomas GN, Tomlinson B, Chan JC, et al. The Trp64Arg polymorphism of the beta3-adrenergic receptor gene and obesity in Chinese subjects with components of the metabolic syndrome. Int J Obes Relat Metab Disord 2000; 24: 545–51
Basile VS, Masellis M, McIntyre RS, et al. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry 2001; 62 Suppl. 23: 45–66
Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res 2011; 217: 337–46
Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care? J Gen Intern Med 2008; 23: 1066–70
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity. Consensus development conference on ntipsychotic drugs and obesity and diabetes.Diabetes Care 2004; 27: 596–600
Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010; 67: 17–24
Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51. Erratum in:Arch Pediatr Adolesc Med 2010; 164:584
Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86: 15–22
Acknowledgements
No funding support was utilized for this article. James Roerig has received a research grant from Eli Lilly and Company. The other authors report no relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roerig, J.L., Steffen, K.J. & Mitchell, J.E. Atypical Antipsychotic-Induced Weight Gain. CNS Drugs 25, 1035–1059 (2011). https://doi.org/10.2165/11596300-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11596300-000000000-00000