Abstract
Increased inflammation and reduced neurogenesis have been associated with the pathophysiology of major depression. Here, we show for the first time how IL-1β, a pro-inflammatory cytokine shown to be increased in depressed patients, decreases neurogenesis in human hippocampal progenitor cells. IL-1β was detrimental to neurogenesis, as shown by a decrease in the number of doublecortin-positive neuroblasts (−28%), and mature, microtubule-associated protein-2-positive neurons (−36%). Analysis of the enzymes that regulate the kynurenine pathway showed that IL-1β induced an upregulation of transcripts for indolamine-2,3-dioxygenase (IDO), kynurenine 3-monooxygenase (KMO), and kynureninase (42-, 12- and 30-fold increase, respectively, under differentiating conditions), the enzymes involved in the neurotoxic arm of the kynurenine pathway. Moreover, treatment with IL-1β resulted in an increase in kynurenine, the catabolic product of IDO-induced tryptophan metabolism. Interestingly, co-treatment with the KMO inhibitor Ro 61-8048 reversed the detrimental effects of IL-1β on neurogenesis. These observations indicate that IL-1β has a critical role in regulating neurogenesis whereas affecting the availability of tryptophan and the production of enzymes conducive to toxic metabolites. Our results suggest that inhibition of the kynurenine pathway may provide a new therapy to revert inflammatory-induced reduction in neurogenesis.
Similar content being viewed by others
INTRODUCTION
The pro-inflammatory cytokine interleukin-1 (IL-1) has been shown to decrease adult hippocampal neurogenesis (Goshen et al, 2008; Koo and Duman, 2008; Kuzumaki et al, 2010). At the same time, increased levels of this cytokine (Capuron and Miller, 2004; Howren et al, 2009; Maes et al, 2009; Miller et al, 2009; Mossner et al, 2007; Raison et al, 2006; Schiepers et al, 2005) as well as reduced levels of hippocampal neurogenesis (Boldrini et al, 2009; Ekdahl et al, 2003; Goshen et al, 2008; Kempermann et al, 2008; Miller et al, 2009) have been reported in depressed patients and in animal models of depression. However, the putative molecular mechanisms linking IL-1-induced inflammation and the reduced neurogenesis that might occur in depression are not well known. Understanding the mechanisms by which inflammatory cytokines give rise to fewer new neurons in the human brain would provide insight that may be used to manage inflammation-associated mental health disorders, including the discovery of novel drug targets for the treatment of depression.
The role of IL-1 in reducing neuronal generation has been repeatedly shown in animal models. Treatment with IL-1 inducers, like lipopolysaccharide (LPS) and radiation, results in marked suppression of hippocampal neurogenesis in rats (Ekdahl et al, 2003; Monje et al, 2003). Moreover, both acute and chronic direct administration of this cytokine decreases hippocampal neurogenesis in rodents (Goshen et al, 2008), and this effect can be blocked by co-administration of an IL-1β receptor antagonist (Koo and Duman, 2008). Additionally, transgenic overexpression of the IL-1 receptor antagonist results in blunted neurogenesis in mice (Spulber et al, 2008). Furthermore, intrahippocampal transplantation of neural precursor cells that overexpress the IL-1 receptor antagonist, therefore blocking IL-1 signalling by chronically elevating levels of the antagonist, completely abolishes the detrimental effect of stress on neurogenesis (Ben Menachem-Zidon et al, 2008).
Interestingly, together with IL-6 and C-reactive protein, IL-1β is a biomarker consistently found to be increased in major depression (Howren et al, 2009). Indeed, depressed patients show an altered peripheral immune system with impaired cellular immunity and increased levels of several pro-inflammatory cytokines, acute phase proteins, chemokines, and cellular adhesion molecules (Howren et al, 2009). We propose that this might be related to the putative reduced neurogenesis in this condition. In particular, levels of IL-1β have been repeatedly described as elevated in depressed patients, both in cerebrospinal fluid (Levine et al, 1999) and in the blood (Anisman and Merali, 1999; Brambilla et al, 2004; Hayley et al, 2005; Owen et al, 2001; Raison et al, 2006; Thomas et al, 2005); moreover, levels appear to be higher in the presence of more severe depressive symptoms (Thomas et al, 2005). At the same time, several lines of evidence suggest the involvement of altered neurogenesis in depression. For example, the birth of new neurons is impaired in stress-induced models of depression in rodents (Malberg and Duman, 2003; Pham et al, 2003), after exposure to acute psychosocial stress in the tree shrew (Gould et al, 1997), and in the brain of depressed patients (Boldrini et al, 2009; Lucassen et al, 2010b); conversely, antidepressants, electroconvulsive therapy and exercise have been shown to enhance neurogenesis (Bjornebekk et al, 2005; Dranovsky and Hen, 2006; Kempermann and Kronenberg, 2003; Lucassen et al, 2010a; Malberg and Duman, 2003; Segi-Nishida et al, 2008; Surget et al, 2008). Therefore, it is possible to speculate that IL-1β may contribute to the reduced neurogenesis in depression. Indeed, mice subjected to chronic stress exhibit increased levels of IL-1β in the hippocampus, together with depressive-like symptoms and reduced hippocampal neurogenesis—both of which are prevented by knockout of the IL-1 receptor(Goshen et al, 2008). Moreover, inhibiting the IL-1 receptor or using IL-1 receptor null mice prevents the anhedonic state and the reduced neurogenesis caused by acute stress exposure (Koo and Duman, 2008). However, although the importance of IL-1β in both depression and neurogenesis is established, the molecular and cellular pathways underlying such effects have not been completely described to date.
One possible downstream molecular mechanism by which IL-1β may reduce neurogenesis is the kynurenine pathway. This pathway, shown in Figure 1, involves conversion of tryptophan into kynurenine, a precursor of both quinolinic acid (QUIN), a potentially neurotoxic metabolite, and kynurenic acid (KYNA), a potentially neuroprotective metabolite. The first step is catalyzed by the enzyme tryptophan-2,3-dioxygenase (TDO) and, especially in response to inflammation, also by indolamine-2,3-dioxygenase (IDO) (Dantzer et al, 2011). This pathway is clearly activated during cytokine-induced depression. For example, administration of LPS in rodents activates IDO and induces depressive-like symptoms, which in turn can be prevented by an IDO antagonist (O’Connor et al, 2009). In the best available clinical model of cytokine-induced depression, treatment with interferon-α, levels of brain kynurenine and QUIN are increased in both blood and cerebrospinal fluid, and correlate with depressive symptoms (Capuron et al, 2002; Raison et al, 2010). Following IDO activation, both the reduced peripheral availability of tryptophan (putatively leading to reduced serotonin synthesis in the brain), and the relative balance between QUIN and KYNA, have been proposed to be of significance in depression and neurodegeneration (Maes et al, 2009; McNally et al, 2008; Muller and Schwarz, 2007; Myint and Kim, 2003; Myint et al, 2007; Wichers et al, 2005). Indeed, TDO-/- mice show increased neurogenesis (Kanai et al, 2009). We therefore propose to test the involvement of the kynurenine pathway in IL-1β-induced reduction in neurogenesis.
Simplified kynurenine pathway of tryptophan metabolism. IDO, indolamine-2,3-dioxygenase; KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase or kynurenine 3-hydroxylase; KYNU, kynureninase; TDO, tryptophan-2,3-dioxygenase; TPH, tryptophan hydroxylase.
We have recently validated a novel in vitro model for depression using human hippocampal progenitor cells (Anacker et al, 2011), where we described how reduced neurogenesis induced by glucocorticoids is reversed by antidepressants. This clinically relevant cellular model of neurogenesis also has the advantage of allowing separate analysis of the proliferation and the differentiation stages. These neural precursors can differentiate into neurons, astrocytes, or oligodendrocytes, but not microglia, which is the cell type most commonly described in immune responses in the brain. In the present study, we use this model to test whether IL-1β affects human neurogenesis, and whether these effects are mediated by the involvement of the kynurenine pathway.
MATERIALS AND METHODS
Cell Culture
We used the multipotent human hippocampal progenitor cell line HPC03A/07 (provided by ReNeuron, Surrey, UK). HPC03A/07 cells were grown in reduced modified media (RMM) consisting of Dulbecco's Modified Eagle's Media/ F12 (DMEM:F12, Invitrogen, Paisley, UK) supplemented with 0.03% human albumin (Baxter Healthcare, Compton, UK), 100 μg/ml human apo-transferrin, 16.2 μg/ml human putrescine DiHCl, 5 μg/ml human recombinant insulin, 60 ng/ml progesterone, 2 mM L-glutamine, and 40 ng/ml sodium selenite. To maintain proliferation, 10 ng/ml human bFGF, 20 ng/ml human EGF, and 100 nM 4-OHT were added.
Where indicated, cells were treated with IL-1β at 10 ng/ml, a dose of ≈0.5 nM, within the range of the IL-1 receptor I affinity (Dinarello, 1996), also previously used in in vitro studies (Koo and Duman, 2008). The kynurenine 3-monooxygenase (KMO) inhibitor Ro 61–8048 was used at 10 μM, after a pilot study where we tried 1, 10, and 100 μM, within the 0.1–100 μM range previously reported to reduce QUIN formation (Chiarugi and [45]Moroni, 1999). In our model, 100 μM caused cell death, whereas 10 μM showed the strongest effect. The IDO inhibitor 1-methyltryptophan (1MT) was used at 500 μM, with higher concentrations causing cell death.
Differentiation Assays
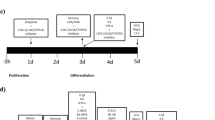
To assess changes in neuronal differentiation, HPC03A/07 cells were plated on blacksided 96-well plates (Nunclon, Roskilde, Denmark) at a density of 1.1. × 104 cells per well. HPC03A/07 cells were allowed to adhere to the bottom of the plate for 24 h, and cultured in the presence of EGF, bFGF, and 4-OHT for 3 days. After this initial proliferation phase, cells were washed and cultured in media without growth factors or 4-OHT for 7 subsequent days. Cells were treated during the initial proliferation phase and the subsequent differentiation phase (total treatment of 10 days) with IL-1β (10 ng/ml), and/or the IDO inhibitor 1-methyltrptophan (1MT, 500 μM) or the KMO inhibitor Ro 61–8048 (10 μM). At the end of the total incubation time (10 days), cells were rinsed with warm phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature (RT). Five independent experiments were conducted on five independent cultures.
Proliferation Assays
To assess progenitor cell proliferation, HPC03A/07 cells were seeded into clear 96-well plates (Nunclon) at a density of 1.1 × 104 cells per well in 200 μl of RMM and incubated for 24 h. After this period, media was changed to start treatment, and cells were cultured for a further 3 days. Cells were treated with IL-1β (10 ng/ml) and/or the KMO inhibitor Ro 61–8048 (10 μM). The synthetic nucleotide 5′-bromodeoxyuridine (BrdU, 10 μM) was added to the culture media 4 h before the end of the incubation period. BrdU incorporates into newly synthesized DNA of dividing cells during the S phase of the cell cycle and allows the monitoring of cell proliferation using immunocytochemistry. At the end of the total incubation time, cells were rinsed with warm PBS and fixed with 4% PFA for 20 min at RT.
In addition, Ki67 expression was also analyzed. Ki67 is a cell cycle and mitosis-related nuclear protein expressed during all phases of the cell cycle except G0 (Scholzen and Gerdes, 2000). In this case, cells were treated for 3 days, rinsed with PBS and fixed with PFA as described above.
Immunocytochemistry
For differentiation assays
Neuronal differentiation was assessed with doublecortin (Dcx) and microtubule-associated protein-2 (MAP2). Briefly, PFA-fixed cells were incubated in blocking solution (10% normal goat serum (NGS), Alpha Diagnostics, San Antonio, TX) in PBS containing 0.1% Triton-X for 2 h at room temperature, and with primary antibodies (rabbit anti-Dcx, 1 : 1000; mouse anti-MAP2 [HM], 1 : 500, Abcam, Cambridge, UK) at 4 °C overnight. Cells were incubated sequentially in blocking solution for 30 min, secondary antibodies (Alexa 594 goat anti-rabbit; 1 : 1000; Alexa 488 goat anti-mouse, 1 : 500, Invitrogen) for 2 h, and Hoechst 33342 dye (0.01 mg/ml, Invitrogen) for 5 min at RT. The number of Dcx and MAP2 positive cells over total Hoechst 33342 positive cells was counted in an unbiased setup with an inverted microscope (IX70, Olympus, Hamburg, Germany) and using ImageJ 1.41 software. Four wells were analyzed per treatment condition in each experiment and three random, non-overlapping pictures were analyzed for each well.
For proliferation assays
Fixed cells were treated with 2N HCL for 15 min to denature the DNA structure of the BrdU incorporated cells, followed by a combined permeabilization (0.1% Triton X-100) in PBS, and blocking step (10% NGS, Sigma) for 1 h at RT. Cells were further incubated overnight at 4 °C with 50 μl rat anti-BrdU (1 : 500, Serotec, Oxford, UK) in PBS with 10% NGS and subsequently washed twice. They were then permeabilized and blocked again for 30 min at RT (0.1% triton X-100 and 10% NGS in PBS) followed by incubation of a rat anti-goat secondary fluorescent antibody (Alexa 488 goat anti-rat, 1 : 500, Invitrogen) for 2 h at RT. The cells were then washed twice with PBS and counterstained with the nuclei Hoechst 33342 (0.01 mg/ml, Invitrogen) for 5 min at RT. Cells were stored in 0.05% sodium azide (Sigma) in PBS at 4 °C until microscopy was ready to be conducted.
In those experiments where BrdU was not used, proliferating cells were examined with Ki67 (rabbit anti-Ki67; 1 : 400, Abcam), and detected with a secondary antibody (Alexa 594 goat anti-rabbit; 1 : 1000, Abcam) followed by the procedure described above for differentiation assays.
RNA Isolation and cDNA Synthesis
Cells at 60–70% confluence in 6-well plates were treated for either 24 h under proliferation conditions or for a total of 10 days (3 days under proliferation conditions followed by 7 days of differentiation conditions), with IL-1β at 10 ng/ml. The RNA was isolated using the RNeasy Micro Kit (Qiagen, Crawley, UK) following the manufacturer's instructions. The extracted RNA was DNAase treated using the Ambion Turbo DNA-free kit (Applied Biosystems) to remove any genomic DNA contamination, and subsequently RNA quality and quantity were assessed using a Nanodrop spectrometer (NanoDrop Technologies, Wilmington, NC). Samples were kept frozen at −80 °C until further use. Subsequently, 1 μg of RNA was used for cDNA synthesis using Superscript III Reverse Transcriptase enzyme according to manufacturer's instructions (Invitrogen), followed by gene expression analyses by quantitative real-time PCR (qPCR).
qPCR
qPCR was performed using HOT FIREPol EvaGreen qPCR Mix (Solis BioDyne, Tartu, Estonia) according to the SYBR Green method and by using Chromo 4 DNA engine, BioRad. For each target primer set, a validation experiment was performed to demonstrate that PCR efficiencies were within the range of 90–100% and equal to the efficiencies of the reference genes. The expression of the target genes IDO, KAT-I, KAT-II, KAT-III, KMO, KYNU, and TDO was normalized to the expression levels of the housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and beta-actin (ACTB) as references. The relative expression levels were quantified using the Pfaffl method (Pfaffl, 2001) and data was normalized to the geometric mean of the housekeeping genes and expressed as fold change compared with the control sample. Three independent experiments were conducted on three independent cultures, and each sample was tested in duplicates.
Ultra Performance Liquid Chromatography/Tandem Mass Spectrometry (UPLC/MS) Method for Tryptophan and Kynurenine Analyses
The analyses of tryptophan and kynurenine were carried out using UPLC/MS. The samples together with internal standard were extracted using solid-phase extraction with methanol, Milli Q water, and 0.1 M citric acid on solid-phase column (Oasis MCX 1 cc) and eluted with elution buffer containing 1 : 2 v/v of tert-butylmethylether and acetonitrile with 5% NH4OH. The eluent was analyzed first by separation using UPLC (Water AQUITY UPLC system) with HSST3 column with gradient elution with two mobile phase solutions (Solvent A 0.1% acetic acid in water; Solvent B 0.1% acetic acid in methanol). The Waters Xevo quadropole MS equipped with electrospray ionization (ESI) was used for analytical detection. The ESI source was set in positive ionization mode. Quantification was performed using different MRM for transition and scan time for different metabolites. Nitrogen was used as desolvation, and cone gas and argon was used as collision gas. Different collision energy was used for different metabolites. All data are collected in multi-channel analysis mode and processed using Mass Lynx version 4.1 software with Target Lynx version 4.1 programme (Waters Corp., Milford, MA).
Drugs
All drugs and reagents were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated. IL-1β and growth factors EGF and bFGF were purchased from Peprotech (London, UK). Ro 61–8048 was purchased from Tocris Bioscience, dissolved in dimethylsulfoxide and aliquots stored at −20 °C. BrdU was dissolved in PBS fresh before use.
Statistical Analysis
Data are presented as mean±SEM. All statistical analyses were performed with GraphPad Prism 4.03 on three or more independent biological replicates (indicated as n). One-Way ANOVA with Newman-Keuls post hoc test was used for multiple comparisons among treatment groups. Student's t-test was used to compare means of two independent treatment groups. p-values <0.05 were considered significant.
RESULTS
IL-1β Decreases Neuronal Differentiation of Human Hippocampal Progenitor Cells
To investigate the effects of IL-1β on neuronal differentiation, we treated cells for 72 h during proliferation, followed by 7 subsequent days of differentiation. Using exactly this experimental paradigm, we have previously shown that antidepressants increase neuronal differentiation in this model (Anacker et al, 2011). Upon addition of IL-1β at 10 ng/ml, we found a decreased number of Dcx-positive cells (−28%). Dcx is a neuronal microtubule-stabilizing protein that characterizes newly generated neuroblasts. To test if IL-1β affected neuronal maturation, the expression of MAP2, which specifically detects more mature neurons, was also analyzed. We found that the number of MAP2-positive cells was also reduced (−36%, Figure 2). This is the first time that the effect of IL-1β on neuronal differentiation is shown in human hippocampal cells, and is consistent with previous observations in rats (Koo and Duman, 2008).
(a) IL-1β reduces differentiation and neuronal maturation of HPC03A/07 human hippocampal progenitor cells. When cells were treated for 3 days during proliferation followed by 7 days under differentiating conditions, IL-1β (10 ng/ml) reduced the amount of Dcx-positive neuroblasts by 28% and the amount of MAP2-positive neurons by 36% compared with the corresponding vehicle treated control. Data are shown as mean±SEM, *p<0.05, **p<0.01. (b) Immunocytochemistry (ICC) showing Hoechst, Dcx, and MAP2 staining. Five independent experiments were conducted on five independent cultures (n=5), four wells were analyzed per treatment condition in each experiment and three random, nonoverlapping pictures were analyzed for each well.
Of note, and in agreement with the above results, we also confirmed that these multipotent precursor cells constitutively express the IL-1 receptor I and, interestingly, also synthesize transcripts for IL-1β, as detected by PCR (data not shown).
IL-1β Modulates the Kynurenine Pathway in Differentiating Human Hippocampal Progenitor Cells
One of the specific objectives of this study was to test whether IL-1β can regulate the kynurenine pathway (see Figure 1 for description of the pathway). We found that differentiated human hippocampal progenitor cells indeed constitutively express both IDO and TDO, the enzymes that metabolize tryptophan into KYN. Moreover, we found that treatment with IL-1β (10 ng/ml) greatly upregulated the levels of transcripts for IDO (42-fold increase, Figure 3a, left three columns) under the conditions described above, that is, after 3 days of culture under proliferating conditions followed by 7 days under differentiation conditions. In contrast, no significant changes were observed in the levels of TDO expression (Figure 3a).
Modulation of mRNA expression levels of (a) IDO, TDO, KMO, and KYNU; (b) KAT1, KAT2, and KAT3, after stimulation with IL-1β (10 ng/ml) for 3 days of proliferation followed by 7 days of differentiation. Results are expressed as fold change vs untreated control cells (n=3). Data are shown as mean±SEM, *p<0.05, **p<0.01, ***p<0.001.
To test whether the IL-1β-induced increased levels of transcripts for IDO translated into functional enzymatic activity, we measured the levels of both tryptophan and kynurenine in the supernatants of culture. As expected, results showed that tryptophan levels decreased from 7.4±0.9 to 5.7±0.6 μg/ml (−19%), and kynurenine levels increased from 69±12 to 83±16 ng/ml (+20%), leading to an overall kynurenine/tryptophan ratio increase of 53±13% (p<0.05), supporting the notion that IL-1ß increases the levels of a functional IDO enzyme.
We also found that IL-1β, in differentiating cells, activates the neurotoxic branch and inhibits the neuroprotective branch of the kynurenine pathway. Specifically, treatment with IL-1β upregulated the levels of transcripts for both enzymes that act upon the neurotoxic branch of the kynurenine pathway: KMO, which showed a 12-fold increase, and kynureninase (KYNU), which showed a 30-fold increase (Figure 3a, right two columns). We also measured the levels of three different KAT isoforms, KAT-I, KAT-II, and KAT-III, which catalyze the transformation of kynurenine into the neuroprotective KYNA. Upon treatment with IL-1β, KAT-I, and KAT-III were downregulated (0.6- and 0.5-fold), whereas the downregulation of KAT-II was smaller (0.9-fold and not significant) (Figure 3b). Although the specific contribution of each KAT isoform in the human brain remains unknown (Han et al, 2010), our overall results suggest that the production of the neuroprotective KYNA may be impaired upon treatment with IL-1β. Unfortunately levels of KYNA could not be detected in any of the supernatants analyzed, possibly because of the detection limit of the technique used for this evaluation (4 ng/ml).
KMO Inhibition Reverses IL-1β-Induced Reduction in Neurogenesis
KMO is the first enzyme in the neurotoxic side arm; since it was upregulated by IL-1β (see above), we wanted to test whether its activation was involved in the IL-1β-induced reduction of neurogenesis. Therefore, we co-treated cells with IL-1β and the KMO inhibitor Ro 61–8048 (10 μM). After 3 days of co-treatment under proliferating conditions followed by an additional 7 days under differentiating conditions, we observed a partial reversion of the reduction in differentiation induced by IL-1β (Dcx: from −42% with IL-1β alone to −26% with IL-1β+ inhibitor; MAP2: from −37% with IL-1β alone to −22% with IL-1β + inhibitor, Figure 4). These results support the notion that the kynurenine pathway is involved in the reduction of neurogenesis caused by this cytokine.
KMO inhibition effects on neuronal differentiation. Co-treatment with IL-1β (10 ng/ml) and the KMO inhibitor Ro 61–8048 (10 μM) caused a partial reversal of the reduction of differentiation and neuronal maturation caused by the cytokine. Dcx: from −42% with IL-1β alone to −26% with IL-1β/inhibitor; MAP2: from −37% with IL-1β alone to −22% with IL-1β/inhibitor. Results are expressed as percentage change vs the corresponding vehicle treated control (n=4). Data are shown as mean±SEM, *p<0.05, **p<0.01.
We also wanted to analyze if interfering with the first step of the pathway would be effective in ameliorating the detrimental effects of IL-1β. Therefore, we treated the cells with the IDO inhibitor, 1MT. We found that at the highest concentration that did not affect viability of cells, 500 μM, 1MT had no effect on the proportion of Dcx (from −25 to −29%, p=0.55) or MAP2 positive cells (from −44 to −62%, p=0.21). Previous observations had reported the in vitro use of 1MT at higher concentrations (1 mM reported by El Kholy et al, 2011; 2 mM reported by Hwang et al, 2005), so it is possible that 500 μM was not an adequate concentration, but unfortunately 1 mM of 1MT caused cell death in our model. Moreover, because of the fact that 1MT is the internal standard used in our HPLC measurements, we were not able to measure levels of kynurenine and tryptophan under these experimental conditions, and so we could not check whether we were indeed inhibiting IDO enzyme activity completely.
IL-1β Increases Human Hippocampal Cell Proliferation
In the experiments described above, we analyzed the effects of IL-1β on neuronal differentiation. In order to investigate the effects on proliferation, we treated progenitor cells with IL-1β for 3 days in the presence of growth factors. As in our previous study on antidepressants (Anacker et al, 2011), we added the synthetic nucleotide BrdU (10 μM) to the culture media for the last 4 h of incubation. Treatment with IL-1β at 10 ng/ml caused an increase in the number of BrdU-positive cells (+23%; Figure 5). Confirmation was obtained by Ki67 immunostaining, which showed the same effect: IL-1β at 10 ng/ml increased the number of Ki67 positive proliferating progenitor cells (+11%, data not shown). This is consistent with some (Seguin et al, 2009) but not all (Koo and Duman, 2008; Wang et al, 2007) previous studies (see also discussion). Interestingly, co-treatment of cells with IL-1β and the KMO inhibitor Ro 61–8048 (10 μM) had the same effect on the number of BrdU-positive cells as that of the cytokine alone (+22% for both, Figure 5), suggesting that the different effects of IL-1β on differentiation vs proliferation are mediated by different mechanisms.
Effect of IL-1β and KMO inhibitor on cell proliferation: (a) Treatment with IL-1β alone or together with the KMO inhibitor Ro 61-8048 (10uM) increased the number of BrdU-positive cells by 23 and 22%, respectively, compared with the corresponding vehicle treated control (one-way ANOVA, **p=0.0047, F=6.13). (b) Immunocytochemistry showing Hoechst and BrdU staining. Three independent experiments were conducted on three independent cultures (n=3), four wells were analyzed per treatment condition in each experiment and three random, nonoverlapping pictures were analyzed for each well.
IL-1β Modulates the Kynurenine Pathway in Proliferating Human Hippocampal Progenitor Cells
The last objective was to test whether the changes we observed in the levels of kynurenine pathway enzymes in differentiating cells (see above) were already present in proliferating cells.
Non-stimulated progenitor cells had a very low basal expression of IDO. Upon treatment with IL-1β (10 ng/ml) for 24 h we found a vast increase in IDO expression levels (approximately 15000-fold), whereas no significant changes were observed for the expression of TDO (Figure 6a, right three columns). Treatment of cells with increasing amounts of IL-1β (from 0.001 ng/ml to 10 ng/ml) showed that the IDO induction was dose-dependent (data not shown).
Modulation of mRNA expression levels of (a) IDO, TDO, KMO, and KYNU; (b) KAT1, KAT2, and KAT3, after stimulation with IL-1β (10 ng/ml) for 3 days of proliferation followed by 7 days of differentiation. Results are expressed as fold change vs untreated control cells (n=3) Data are shown as mean±SEM. *p<0.05, **p<0.01, ***p<0.001.
Finally, in proliferating cells IL-1β activated both the neurotoxic branch (but less than in differentiating cells) and the neuroprotective branch of the kynurenine pathway. This was different from what we showed above in differentiating cells, where IL-1β only activated the neurotoxic branch and inhibited the neuroprotective branch. Specifically, IL-1β upregulated the level of transcripts for KMO and KYNU (4- and 10.4-fold increase, respectively, Figure 6a, right two columns) in proliferating cells. Compared with the 12- and 30-fold upregulation, respectively, in differentiating cells (see above), the upregulation in proliferating cells was much smaller. Moreover, we found that KAT-II was significantly upregulated (1.5-fold increase, Figure 6b). Furthermore, although not significant, a trend towards increased levels was observed in the expression of both KAT-I and KAT-III (1.4- and 1.3-fold, respectively). This is in contrast with the downregulation of these three enzymes in differentiating cells (see above).
DISCUSSION
Our results show for the first time that IL-1β reduces human hippocampal neurogenesis whereas activating the neurotoxic branch of the kynurenine pathway. In addition, we show that IL-1β promotes proliferation of undifferentiated progenitor cells, a condition that is associated with increased activation of the neuroprotective branch of the kynurenine pathway.
We demonstrate here that IL-1β exerts a negative effect on human hippocampal neurogenesis, as shown by a decrease in the number of both Dcx-positive neuroblasts (−36%), and more mature, MAP2-positive neurons (−28%). Our data is in line with previous observations in mice, where chronic exposure to IL-1β results in decreased numbers of Dcx-positive new neurons (Goshen et al, 2008). A similar role for IL-1β is shown indirectly in rats, as administration of an IL-1 receptor antagonist results in blockade of the decreased levels of neurogenesis observed when they are subjected to acute stressors (Koo and Duman, 2008). Moreover, administration of LPS, a strong activator of microglia and inducer of IL-1β, causes reduction in neurogenesis. However, another study found no effects when rat hippocampal progenitor cells were left to differentiate in vitro in the presence of IL-1β (Monje et al, 2003). Considering that neurogenesis has been implicated in both depression and cognitive function (Kempermann et al, 2004), and in view of additional inconsistencies observed in different animal species on proliferation (described below), our results showing that IL-1β exerts detrimental effect on human neurogenesis is particularly relevant.
Multiple steps are involved in neurogenesis, including proliferation, migration, differentiation, survival, and integration of the newly formed neurons into the circuitry of the CNS (Ming and Song, 2005). In our study, we demonstrated that IL-1β, which is present continuously in the culture media during 3 days, promotes proliferation of progenitor cells, as shown by an increase in BrdU incorporation (+19%). However, several and quite diverse effects on proliferation have been reported for IL-1β. For example, although one study showed that proliferation of embryonic rat neural precursors was reduced by IL-1β in a dose-dependent manner, in part because of IL-1β induced apoptosis (Wang et al, 2007), in another study i.c.v. treatment of rats resulted in decreased hippocampal cell proliferation, which was not accounted for by apoptosis (Koo and Duman, 2008). Furthermore, yet another study in mice showed increased cell proliferation after repeated but not single intra-hippocampal infusion with IL-1β, whereas no effects were observed if the administration was systemic (Seguin et al, 2009). Moreover, hippocampal proliferation was increased in adolescent but not older mice in response to IL-1α, which acts through the same IL-1R1 receptor as IL-1β (McPherson et al, 2011). Therefore, these studies show that the effects of IL-1β on cell proliferation are dependent on species, age, route, and length of administration. It is important to emphasize that although proliferation, ie, self renewal of the unspecialized stem cells is increased by IL-1β, neuronal differentiation, ie development of such unspecialized cells into neurons, is decreased. Therefore, the increase in cell proliferation does not result in more neurogenesis, as it does not actually contribute to the addition of more (functionally active) neurons to the hippocampal circuitry. Similar differential effects on proliferation vs differentiation have previously been observed in these cells upon antidepressant treatment (Anacker et al, 2011). As mentioned above, these neural precursors can differentiate into neurons, astrocytes or oligodendrocytes, and it will be interesting for future studies to investigate the effects of IL-1β on differentiation of precursors into other cell types, as well as, the functional relevance of proliferation vs differentiation for hippocampal function and behavior.
In order to clarify the mechanisms by which IL-1β could reduce neurogenesis, we focused the attention on the kynurenine pathway. As shown in Figure 1, the pathway starts with two different enzymes, TDO and IDO, which initiate the catabolism of tryptophan towards kynurenine. Although TDO is induced by tryptophan and metabolic steroids, IDO is known to be induced by LPS and pro-inflammatory cytokines during an immune response (Wirleitner et al, 2003). Although the physiological purpose of these different regulatory mechanisms remains a matter of controversy, two pieces of evidence are relevant to our study: first, TDO−/− mice, which have increased tryptophan levels in the hippocampus, show increased neurogenesis together with anxiolytic effects (Kanai et al, 2009), and; second, dysregulation of this pathway has been widely implicated in inflammation-mediated depression (Dantzer et al, 2008). Specifically, this dysregulation in depression involves activation of IDO and concomitant increased levels of kynurenine, which can further progress into either KYNA or through 3-hydroxykynurenine (3-HK) towards QUIN. Although KYNA is an N-methyl D-aspartate (NMDA) antagonist considered potentially neuroprotective (Moroni, 1999; Nozaki and Beal, 1992), both 3-HK and QUIN are considered neurotoxic: 3-HK acts as an oxidative stress generator releasing free hydroxyl radicals (Eastman and Guilarte, 1990) and QUIN is an NMDA receptor agonist, associated with excitotoxicity (Schwarcz et al, 1983; Stone and Perkins, 1981). As the relative balance between these metabolites has been suggested to contribute to the pathophysiology of depression (McNally et al, 2008; Muller and Schwarz, 2007; Wichers et al, 2005), we were interested in looking at the effects of IL-1β on the expression levels of all enzymes that regulate their production within the kynurenine pathway, in differentiating cells. We found that IL-1β induced an upregulation of transcripts for IDO (42-fold increase), together with an upregulation of both enzymes involved in the neurotoxic branch, KMO and KYNU (12- and 30-fold increase, respectively). Interestingly, KAT1, KAT2, and KAT3, the enzymes conducive to the potentially neuroprotective KYNA, were downregulated (0.6-, 0.9-, and 0.5-fold, respectively). This evidence is consistent with clinical data showing a significant reduction in KYNA levels in the plasma of drug naïve depressed patients (Myint et al, 2007). Interestingly, some antidepressants have recently been reported as decreasing levels of KMO and increasing levels of KAT1 and KAT2, reestablishing a potentially favorable KYNA/3-HK ratio (Kocki et al, 2011).
We also show that, upon activation of IDO, treatment with IL-1β results in a decrease of tryptophan together with an increase in kynurenine levels. Increased brain levels of kynurenine and QUIN have been reported in cerebrospinal fluid of IFN-α treated patients who develop depressive symptoms as a consequence of the cytokine administration (Raison et al, 2010). Additionally, elevated plasma levels of kynurenine have also been observed in depressed suicide attempters (Sublette et al, 2011). Therefore, our data are consistent with clinical evidence showing an involvement of both IL-1β and the neurotoxic branch of the kynurenine pathway in depression.
Co-treatment of our cells with IL-1β and the KMO inhibitor, Ro 61–8048, results in a partial reversion of the reduction in differentiation induced by IL-1β (Dcx: from −42% with IL-1β alone to −26% with IL-1β/inhibitor; MAP2: from −37% with IL-1β alone to −22% with IL-1β/inhibitor). The most widely used KMO inhibitor, Ro 61-8048 (Rover et al, 1997), has been previously shown to be beneficial in rodent models of brain ischemia (Moroni, 1999). Moreover, it was recently shown that the sustained inhibition of KMO appears to reverse a number of cognitive and motor deficits measured in neurodegeneration, using mouse models of Alzheimer's disease and Huntington's disease (Zwilling et al, 2011). Inhibiting KMO has the effect of elevating neuroprotective KYNA levels whereas decreasing the levels of neurotoxic 3-HK and QUIN (Chiarugi et al, 1995; Moroni et al, 2003). Additionally, further evidence that inhibition of KMO is neuroprotective has been described recently (Campesan et al, 2011) in a fruit fly model of Huntington's disease, a disorder also characterized by elevated inflammatory responses (Hsiao and Chern, 2010). Interestingly, feeding KYNA to Huntington's disease model flies protects them from neurodegeneration, whereas feeding 3-HK exacerbates cytotoxicity. However, there is also evidence of potentially negative effects of KMO inhibition. For example, a recent paper found a reduction in KMO gene expression and KMO enzyme activity in postmortem tissue from the frontal eye field in schizophrenia patients (Wonodi et al, 2011) suggesting that decreased KMO levels may be related to the etiopathophysiology of this disorder. Also, the long-term consequences of an imbalance between the two arms of the kynurenine pathway are unknown. Therefore further experimental and clinical work should be conducted before considering this approach for clinical use. Nevertheless, careful pharmacological normalization of the imbalance in brain kynurenine metabolites may provide clinical benefits in inflammation-induced depression, where alterations of the kynurenine pathway are frequently found.
It is important to discuss some potential limitations of our work. First of all, this is an in vitro neuronal study, and from a translational point of view, in the brain in vivo the glial and microglial environment will have an important role for the fate of differentiating neurons. Additionally, cytokines are pleiotrophic molecules and therefore the local effects in one particular brain region, such as the hippocampus, may have different outcomes on neuronal function in different areas. Moreover, cytokines may regulate brain function by a variety of mechanisms, not only neurogenesis. For example, IL-1β has been shown to cause long-term potentiation deficiency in a mouse model of septic encephalopathy (Imamura et al, 2011), and therefore synaptic plasticity changes because of this cytokine have to be considered in an in vivo setting. Another limitation of our study is that we have measured mRNA levels of enzymes, but have limited data on the functional significance of these changes. For example, we do have an indication of activity of IDO from measuring levels of tryptophan and kynurenine, but we could not detect KA and we could not technically measure other metabolites, including QUIN (potentially the most important neurotoxic metabolite) and picolinic acid, which is also considered neuroprotective (Guillemin et al, 2007). Finally, although we did not find an effect of the IDO inhibitor, 1MT, on neurogenesis, we cannot exclude an involvement of IDO in this process, as we could only use a low concentration of inhibitor (the higher causing cell death in our system) and we could not assess IDO activity after treatment with the inhibitor (since 1MT is also the internal standard for the HPLC detection of metabolites).
In summary, our observations indicate that IL-1β has a critical role in regulating neurogenesis by activating the kynurenine pathway and subsequently affecting the availability of tryptophan and the production of enzymes conducive to neurotoxic metabolites. Inhibition of KMO partially reverts the damaging effects of IL-1β on neurogenesis, suggesting that carefully targeting the kynurenine pathway may provide a new therapy for managing inflammation-associated mental health disorders characterized by decreased neurogenesis and/or an imbalance in the levels of neurotoxic and neuroprotective metabolites.
References
Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S et al (2011). Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 16: 738–750.
Anisman H, Merali Z (1999). Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol 461: 199–233.
Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T et al (2008). Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology 33: 2251–2262.
Bjornebekk A, Mathe AA, Brene S (2005). The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol 8: 357–368.
Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J et al (2009). Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34: 2376–2389.
Brambilla F, Monteleone P, Maj M (2004). Interleukin-1beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. J Affect Disord 78: 273–277.
Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R et al (2011). The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr Biol 21: 961–966.
Capuron L, Miller AH (2004). Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56: 819–824.
Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R (2002). Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 7: 468–473.
Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F (1995). Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem 65: 1176–1183.
Chiarugi A, Moroni F (1999). Quinolinic acid formation in immune-activated mice: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(-3-nitrophenyl)thiazol-2yl]-benzenesul fonamide (Ro 61-8048), two potent and selective inhibitors of kynurenine hydroxylase. Neuropharmacology 38: 1225–1233.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56.
Dantzer R, O’Connor JC, Lawson MA, Kelley KW (2011). Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36: 426–436.
Dinarello CA (1996). Biologic basis for interleukin-1 in disease. Blood 87: 2095–2147.
Dranovsky A, Hen R (2006). Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59: 1136–1143.
Eastman CL, Guilarte TR (1990). The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res 15: 1101–1107.
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100: 13632–13637.
El Kholy NM, Sallam MM, Ahmed MB, Sallam RM, Asfour IA, Hammouda JA et al (2011). Expression of indoleamine 2,3-dioxygenase in acute myeloid leukemia and the effect of its inhibition on cultured leukemia blast cells. Med Oncol 28: 270–278.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T et al (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13: 717–728.
Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997). Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17: 2492–2498.
Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V et al (2007). Characterization of the kynurenine pathway in human neurons. J Neurosci 27: 12884–12892.
Han Q, Cai T, Tagle DA, Li J (2010). Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci 67: 353–368.
Hayley S, Poulter MO, Merali Z, Anisman H (2005). The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 135: 659–678.
Howren MB, Lamkin DM, Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186.
Hsiao HY, Chern Y (2010). Targeting glial cells to elucidate the pathogenesis of Huntington's disease. Mol Neurobiol 41: 248–255.
Hwang SL, Chung NP, Chan JK, Lin CL (2005). Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res 15: 167–175.
Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H et al (2011). Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience 187: 63–69.
Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K et al (2009). Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain 2: 8.
Kempermann G, Krebs J, Fabel K (2008). The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry 21: 290–295.
Kempermann G, Kronenberg G (2003). Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54: 499–503.
Kempermann G, Wiskott L, Gage FH (2004). Functional significance of adult neurogenesis. Curr Opin Neurobiol 14: 186–191.
Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM (2011). New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm (e-pub ahead of print).
Koo JW, Duman RS (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 105: 751–756.
Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M et al (2010). Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse 64: 721–728.
Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V (1999). Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40: 171–176.
Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E et al (2010a). Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol 20: 1–17.
Lucassen PJ, Stumpel MW, Wang Q, Aronica E (2010b). Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58: 940–949.
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G et al (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24: 27–53.
Malberg JE, Duman RS (2003). Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28: 1562–1571.
McNally L, Bhagwagar Z, Hannestad J (2008). Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr 13: 501–510.
McPherson CA, Aoyama M, Harry GJ (2011). Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun 25: 850–862.
Miller AH, Maletic V, Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741.
Ming GL, Song H (2005). Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28: 223–250.
Monje ML, Toda H, Palmer TD (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302: 1760–1765.
Moroni F (1999). Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol 375: 87–100.
Moroni F, Carpenedo R, Cozzi A, Meli E, Chiarugi A, Pellegrini-Giampietro DE (2003). Studies on the neuroprotective action of kynurenine mono-oxygenase inhibitors in post-ischemic brain damage. Adv Exp Med Biol 527: 127–136.
Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N et al (2007). Consensus paper of the WFSBP task force on biological markers: biological markers in depression. World J Biol Psychiatry 8: 141–174.
Muller N, Schwarz MJ (2007). The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry 12: 988–1000.
Myint AM, Kim YK (2003). Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses 61: 519–525.
Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2007). Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98: 143–151.
Nozaki K, Beal MF (1992). Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J Cereb Blood Flow Metab 12: 400–407.
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N et al (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14: 511–522.
Owen BM, Eccleston D, Ferrier IN, Young AH (2001). Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand 103: 226–228.
Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
Pham K, Nacher J, Hof PR, McEwen BS (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17: 879–886.
Raison CL, Capuron L, Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31.
Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G et al (2010). CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15: 393–403.
Rover S, Cesura AM, Huguenin P, Kettler R, Szente A (1997). Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem 40: 4378–4385.
Schiepers OJ, Wichers MC, Maes M (2005). Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29: 201–217.
Scholzen T, Gerdes J (2000). The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322.
Schwarcz R, Whetsell Jr WO, Mangano RM (1983). Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219: 316–318.
Segi-Nishida E, Warner-Schmidt JL, Duman RS (2008). Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci USA 105: 11352–11357.
Seguin JA, Brennan J, Mangano E, Hayley S (2009). Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat 5: 5–14.
Spulber S, Oprica M, Bartfai T, Winblad B, Schultzberg M (2008). Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci 27: 549–558.
Stone TW, Perkins MN (1981). Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol 72: 411–412.
Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA et al (2011). Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun 25: 1272–1278.
Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G et al (2008). Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64: 293–301.
Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT (2005). Increase in interleukin-1beta in late-life depression. Am J Psychiatry 162: 175–177.
Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W et al (2007). Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci 36: 343–354.
Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M (2005). IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10: 538–544.
Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D (2003). Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem 10: 1581–1591.
Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE et al (2011). Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry 68: 665–674.
Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ et al (2011). Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 145: 863–874.
Acknowledgements
This work was supported by a NARSAD award to PAZ; a studentship from the NIHR ‘Biomedical Research Centre for Mental Health’, Institute of Psychiatry and South London and Maudsley NHS Foundation Trust to CA; and a grant from the Commission of European Communities 7th Framework Programme Collaborative Project Grant Agreement n 22963 (Mood Inflame) to CMP and AMM. ST is supported in part by a grant from Research Councils UK. Special thanks to MB Elias for help with illustrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In the last 3 years, CMP has received speaker's fee from Lilly and Roche. JP is a consultant and has received payment from ReNeuron Group. AMM is a consultant at Advanced Practical Diagnostics N.V. in Belgium. PAZ, CA, AC, SC, KM and ST declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zunszain, P., Anacker, C., Cattaneo, A. et al. Interleukin-1β: A New Regulator of the Kynurenine Pathway Affecting Human Hippocampal Neurogenesis. Neuropsychopharmacol 37, 939–949 (2012). https://doi.org/10.1038/npp.2011.277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.277
Keywords
This article is cited by
-
Kynurenine pathway and its role in neurologic, psychiatric, and inflammatory bowel diseases
Molecular Biology Reports (2023)
-
Carnosic Acid Mitigates Depression-Like Behavior in Ovariectomized Mice via Activation of Nrf2/HO-1 Pathway
Molecular Neurobiology (2023)
-
Effect modification of tumor necrosis factor-α on the kynurenine and serotonin pathways in major depressive disorder on type 2 diabetes mellitus
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Involvement of kynurenine pathway between inflammation and glutamate in the underlying etiopathology of CUMS-induced depression mouse model
BMC Neuroscience (2022)
-
Neurogenesis is disrupted in human hippocampal progenitor cells upon exposure to serum samples from hospitalized COVID-19 patients with neurological symptoms
Molecular Psychiatry (2022)