Abstract
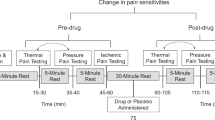
Endogenous opioid and cannabinoid systems are thought to act synergistically regulating antinociceptive and reward mechanisms. To further understand the human implications of the interaction between these two systems, we investigated the role of the common, functional missense variant Pro129Thr of the gene coding fatty acid amide hydrolase (FAAH), the major degrading enzyme of endocannabinoids, on psychophysical and neurotransmitter (dopaminergic, opioid) responses to pain and placebo-induced analgesia in humans. FAAH Pro129/Pro129 homozygotes, who constitute nearly half of the population, reported higher placebo analgesia and more positive affective states immediately and 24 h after placebo administration; no effects on pain report in the absence of placebo were observed. Pro129/Pro129 homozygotes also showed greater placebo-induced μ-opioid, but not D2/3 dopaminergic, enhancements in neurotransmission in regions known involved in placebo effects. These results show that a common genetic variation affecting the function of the cannabinoid system is serving as a probe to demonstrate the involvement of cannabinoid and opioid transmitters on the formation of placebo effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zubieta JK et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005; 25: 7754–7762.
Amanzio M, Benedetti F . Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999; 19: 484–494.
Kogan NM, Mechoulam R . The chemistry of endocannabinoids. J EndocrinolI Invest 2006; 29 (3 Suppl): 3–14.
Hohmann AG . Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids 2002; 121: 173–190.
Gardner EL, Vorel SR . Cannabinoid transmission and reward-related events. Neurobiol Dis 1998; 5 (6 Pt B): 502–533.
Colloca L, Sigaudo M, Benedetti F . The role of learning in nocebo and placebo effects. Pain 2008; 136: 211–218.
Benedetti F, Amanzio M, Rosato R, Blanchard C . Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med 2011; 17: 1228–1230.
Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M . CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport 2001; 12: 3689–3692.
Ledent C et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999; 283: 401–404.
Welch SP . Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry 2009; 21: 143–151.
Haller VL, Stevens DL, Welch SP . Modulation of opioids via protection of anandamide degradation by fatty acid amide hydrolase. Eur J Pharmacol 2008; 600: 50–58.
Wilson RI, Nicoll RA . Endocannabinoid signaling in the brain. Science 2002; 296: 678–682.
Chiang KP, Gerber AL, Sipe JC, Cravatt BF . Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet 2004; 13: 2113–2119.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK . Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 2008; 65: 220–231.
Zubieta JK et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001; 293: 311–315.
De Vries TJ et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 2001; 7: 1151–1154.
Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA . Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol 2005; 15: 31–37.
Hodgkinson CA et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol 2008; 43: 505–515.
Stohler CS, Kowalski CJ . Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain 1999; 79: 165–173.
Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54: 1063–1070.
Pollock V, Cho DW, Reker D, Volavka J . Profile of Mood States: the factors and their physiological correlates. J Nervous Mental Dis 1979; 167: 612–614.
Melzack R, Torgerson WS . On the language of pain. Anesthesiology 1971; 34: 50–59.
Jewett DM . A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nuclear Med Biol 2001; 28: 733–734.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL . Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cerebral Blood Flow Metab 1996; 16: 834–840.
Narendran R, Martinez D . Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 2008; 62: 851–869.
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O . Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci 2000; 12: 4038–4046.
Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD . Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 2006; 50: 507–517.
Pecina M, Stohler CS, Zubieta JK . Role of mu-opioid system in the formation of memory of placebo responses. Mol Psychiatry 2012; 18: 135–137.
Cravatt BF et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 2001; 98: 9371–9376.
Sim LJ, Selley DE, Xiao R, Childers SR . Differences in G-protein activation by mu- and delta-opioid, and cannabinoid, receptors in rat striatum. Eur J Pharmacol 1996; 307: 97–105.
Sim-Selley LJ, Martin BR . Effect of chronic administration of R-(+)-[2,3-Dihydro–5-methyl–3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]–1,4-benzoxaz inyl]-(1-naphthalenyl)methanone mesylate (WIN55,212–2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther 2002; 303: 36–44.
Rice OV, Gordon N, Gifford AN . Conditioned place preference to morphine in cannabinoid CB1 receptor knockout mice. Brain Res 2002; 945: 135–138.
Mas-Nieto M et al. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol 2001; 132: 1809–1816.
Navarro M et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci 2001; 21: 5344–5350.
Di Marzo V . Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 2008; 7: 438–455.
Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH . Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther 2010; 334: 182–190.
Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D . Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212–2. Eur J Neurosci 2007; 25: 2191–2200.
Fattore L et al. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav 2005; 81: 343–359.
Acknowledgements
We wish to acknowledge the nuclear medicine technologists of the PET Center at the University of Michigan for the assistance in PET data acquisition and reconstruction. Funding: work was supported by R01 DA 022520, R01 DA027494 and the Phil F Jenkins Foundation (JKZ). MMJ was supported by the Spanish Ministry of Education (MMJ: AP2008–03742).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
JKZ, CSS and DG were responsible for the study design and procured the study funding; JKZ and CSS collected data; MP, MMJ and CH analyzed the data; MP and JKZ wrote the manuscript.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Peciña, M., Martínez-Jauand, M., Hodgkinson, C. et al. FAAH selectively influences placebo effects. Mol Psychiatry 19, 385–391 (2014). https://doi.org/10.1038/mp.2013.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.124
Keywords
This article is cited by
-
Targeting the endocannabinoid system for the treatment of abdominal pain in irritable bowel syndrome
Nature Reviews Gastroenterology & Hepatology (2023)
-
D3 dopamine receptors and a missense mutation of fatty acid amide hydrolase linked in mouse and men: implication for addiction
Neuropsychopharmacology (2020)
-
Genome-wide association studies of placebo and duloxetine response in major depressive disorder
The Pharmacogenomics Journal (2018)
-
Mechanisms of the placebo effect in pain and psychiatric disorders
The Pharmacogenomics Journal (2016)
-
Words and Drugs: Same Mechanisms of Action?
Journal of Contemporary Psychotherapy (2016)