Article Text
Abstract
Major depressive disorder (MDD) is a common, serious, debilitating condition affecting 350 million people worldwide, which remains to be unsatisfactorily treated with 53% of patients still complaining of symptoms after completing their courses with the correct dosage. Ketamine, which was approved by the Food and Drug Administration in 2019, is a potential treatment option for those recalcitrant cases. The mechanism of ketamine is not fully understood, but as type it is classified as an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, and can be given intravenously, intranasally and orally. It is used to treat treatment-resistant depression, depression associated with suicidal ideation, mood and anxiety disorders and depressions associated with either type of bipolar disorder. Although ketamine is considered relatively safe, several side effects have been reported with the major ones being psychiatric in the form of worsening mood, anxiety and agitation; psychotomimetic in the form of dissociation, perceptual disturbance and abnormal sensations; cardiovascular in the form of increased blood pressure and increased heart rate; and neurological in the form of headache and dizziness. Ketamine is still not approved worldwide for usage in patients with treatment-resistant MDD, but if it is approved sometime in the future with relatively fewer side effects, it is expected to significantly save millions of dollars spent yearly on patients with treatment-resistant depression and that will lift this major burden off the shoulders of healthcare professionals. This study was designed to measure the effects of ketamine, an NMDA receptor antagonist, on patients with treatment-resistant MDD and to analyse the concept that makes it different and relatively safer than other major antidepressants like selective serotonin reuptake inhibitors, monoamine oxidase inhibitors and TCAs (tricyclic antidepressants).
- depressive disorder, treatment-resistant
- anxiety
- bipolar disorder
- depressive disorder, major
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Major depressive disorder (MDD) is a serious condition that affects the lives of many individuals globally. It is estimated that the total number of cases diagnosed with MDD is 350 million people around the world.1 2 And eight hundred thousand patients die every year due to suicidal ideation caused by MDD. MDD poses major burdens to the healthcare systems of different countries around the world.1–3 Despite the different treatment options available in the form of selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants (TCA) and monoamine oxidase inhibitors (MAOI), none of them were capable of completely relieving the symptoms of depression and many cases have reported frequent relapses, almost 53%, after completing the courses of their medications with the appropriate doses2 with 20% failing to show improvement on usage of subsequent lines of therapy.2 4 The current economic burden of MDD in the USA alone is estimated at $29–$48 billion per year.5 In fact, it carries a major economic burden in the form of the requirement of numerous emergency rooms, lacking of responsiveness to the current medications and major unemployment to many healthcare professionals.2 6
Currently, there is no consensus on the definition of treatment-resistant depression (TRD), but the current definition is that it is any form of depression in which the patient is not fully relieved from the major symptoms of depression after taking the appropriate courses of at least two antidepressant medications at their adequate time duration.2–5 The main limitation in the usage of these agents, besides the lack of appropriate responsiveness, is the fact that they could take more than 2–4 weeks in order for the initial response to be felt by the patients; in addition numerous studies have shown that many patients report multisystemic, unbearable side effects.7 8
In March 2019, the US Food and Drug Administration (FDA) approved a new antidepressant medication under the name of ketamine, an N-methyl-D-aspartate (NMDA) type of glutamate receptor antagonist. This review is dedicated to knowing the pharmacological details of this medication and how it is different from other antidepressants in the management of TRD.7 8
Ketamine is not a perfect drug. Although studies in many patients have been shown to produce positive results in patients with TRD, they have shown that significant side effects have caused many cases to cease undergoing drug trials. Especially patients with chronic cardiovascular conditions like myocardial infarction, arteriovenous fistulae and chronic hypertension should not be allowed to use ketamine.
Uses and dosage
Ketamine is an NMDA receptor antagonist that is used in the management of TRDs, depression associated with suicidal ideation, mood and anxiety disorders and depressions associated with either type of bipolar disorder. Unlike other NMDA antagonists, like memantine that is used in the symptomatic management of Alzheimer’s disease, the mechanism by which ketamine deals with depression follows a different pathway. It is not fully understood how ketamine treats depression, but it works by different mechanisms. Ketamine could be given by three different methods: orally, intravenously and nasally.9 10
Intravenously
The majority of the randomised controlled trials using a dose of 0.5 mg/kg over 40 min of intravenous ketamine followed by a 20% saline infusion washout have shown significant improvement in depression symptoms and suicidal ideation within 1 week as measured using the Montgomery and Asberg Depression Rating Scale (MADRS).11 12 Response rates for patients using intravenous ketamine were shown to be 50%–70%, which proves to be better than other classes of antidepressants.13 14 Most of the patients have not reported any serious side effects12; however, some patients decided to stop taking the drug after showing major side effects.
Intranasal
The US FDA has recently approved the usage of intranasal esketamine along with an oral antidepressant. The doses used in most of the studies were an initial dose of 28 mg/kg and a maintenance dose of 56 or 84 mg/kg. Most cases have statistically shown significant improvements over 40 min according to the MADRS score. Some major side effects were shown in some patients mainly because of the high level of bioavailability of esketamine as it has no first-pass metabolism.15
Those side effects included dissociation, fatigue, poor memory, severe headaches, nausea, elevated heart rate and blood pressure.
Oral
Oral ketamine undergoes a high level of the first-pass metabolism which makes its bioavailability less than 20%. A small open-label study of oral ketamine with a dose of 0.5 mg/kg in hospice patients for 28 days has shown a significant improvement in the MADRS depression scale.16–18 The time to response was 1–2 weeks; however, some patients have decided to withdraw from the trial due to lack of response. Dizziness, delirium, hallucinations, nausea, vomiting and headache were the reported side effects in most of the studies.9
Mechanism of action
Currently, there is no full consensus among authors on the exact mechanism/s by which ketamine plays a role in the management of TRD. Basically, ketamine is an antagonist that blocks the NMDA receptors, but evidence based on numerous studies has proven that the rapid antidepressant (RAAD) effect that ketamine establishes is not based on its NMDA receptor antagonism. While other FDA-approved antidepressants have their antidepressant roles derived from their effects on monoamines such as serotonin, norepinephrine and dopamine, the emergence of ketamine has provided a new perspective that is based on its effects on the glutamate system providing RAAD effects within 4 hours with a long-term effect that lasts for 1 week after a single dose.19
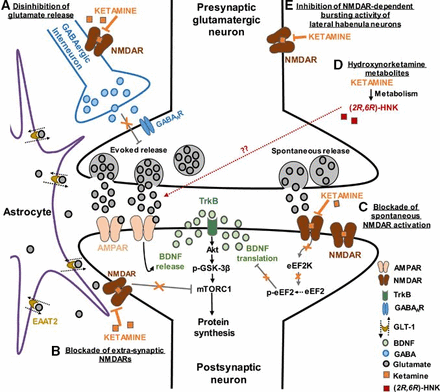
Numerous publications have stated that ketamine induces synaptogenesis at the level of the prefrontal cortex, a part of the brain that atrophies in depressed patients.20 Other studies have also shown that ketamine upregulates the activity of a certain type of receptors called glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) which plays an important role in increasing the levels of the mammalian target of rapamycin (mTOR) which induces synaptogenesis at the level of the prefrontal cortex.21 22 Some authors have come to merge the two hypotheses, as shown in figure 1, concluding that the NMDA receptor antagonistic effect of ketamine on GABAergic interneurons induces the release of glutamate from the prefrontal cortex which in turn activates the AMPA receptors. This activation has been shown to urge the release of brain-derived neurotrophic factor and vascular endothelial growth factor, both of which act on inducing the mTOR, which induces synaptogenesis at the prefrontal cortex level.23–25 This cycle is thought to be relevant to the antidepressant effect of ketamine.
Ketamine works by two currently merged mechanisms: N-methyl-D-aspartate (NMDA) receptor and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor mechanisms. The NMDA receptor antagonistic effect of ketamine on GABAergic interneurons induces the release of glutamate from the prefrontal cortex which in turn activates the AMPA receptors. AMPAR, AMPA receptor; BDNF, brain-derived neurotrophic factor; GABA, gamma-aminobutyric acid; GLT-1, glutamate transporter 1; NMDAR, NMDA receptor.
Side effects
Side effects reported from 60 studies have shown a variety of adverse effects affecting various body systems: psychiatric, cardiovascular, neurological, cognitive and others. Those studies involved 899 patients who received at least one dose of ketamine.26 Psychiatric side effects were described in 38% of the studies, whereas psychotomimetic ones were described in 72%. The psychiatric side effects included most commonly anxiety, followed by agitation and irritability, delusions and mood elevation. Less common side effects included detachment, emotional blunting, psychosis, craving attention and formal thought disorder. Only one case of suicidal attempt was reported in one study.27 The most commonly reported side effects from most studies that led the participants to withdraw from the trial included worsening mood (12 participants), anxiety (6 participants) and suicidal ideation (5 participants). The psychotomimetic side effects most commonly included dissociation, perceptual disturbance, odd or abnormal sensation, derealisation, hallucinations and depersonalisation. The only cases reported to withdraw from the trials were the ones who suffered dissociation.28 29 Most studies that used the intravenous route of administration rather than the other routes were the ones who mostly had psychiatry and psychotomimetic side effects. WHO has classified ketamine as a Schedule III drug as it carries a potential risk for addiction and abuse. It can be abused in any administered form, intravenously, orally or nasally. Ketamine has a potential risk of abuse by patients self-medicating themselves from depression.
Thirty-eight percent of the 60 studies reported cardiovascular side effects. Most of these changes were in the form of increased blood pressure, increased heart rate, arrhythmia, palpitations, dizziness, chest tightness and decreased blood pressure on standing. Only five cases withdrew from the studies due to serious cardiovascular effects. Most of those effects were also reported on the usage of intravenous ketamine infusion. Those effects only lasted 90 min reportedly.
The most commonly reported neurological side effects were headache and dizziness. The most commonly reported cognitive side effects included poor memory, poor concentration, confusion and cognitive impairment.
Multiple other side effects were reported in 32 (53%) of the studies, these side effects were mainly gastrointestinal, ocular, respiratory and urological.
The most frequently reported other side effects included blurred vision and nausea. Less commonly reported ones were insomnia, general malaise, fatigue, restlessness, dry mouth, vomiting and tearfulness.27
Drug–drug interactions
Certain types of drugs should not be used concomitantly with any form of ketamine. Some of these drugs can interfere with the first-pass metabolism in case of being used with oral or intravenous ketamine, others may cause cardiovascular problems especially in individuals with cardiovascular diseases. Such drugs include benzodiazepines, antipsychotics, opioids and alcohol which may all cause sedation. Blood pressure and heart monitoring should be measured in case of using drugs like MAOIs, TCAs, SSRIs, modafinil, armodafinil and amphetamine types of drugs.30 Clonidine can be used safely with esketamine as it can reduce esketamine’s ability to cause hypertension and other side effects.31 Midazolam reduces the sympathomimetic effects of ketamine.32 Certain CYP(Cytochrome P450) enzyme inducers like rifampicin can reduce the concentration of esketamine and norketamine by 10% and 50%, respectively.33 Coadministration of ketamine and ticlopidine has been proven to increase the maximum plasma concentration of ketamine by 72% and total hepatic clearance by 15.5%.34
Conclusions
Since getting approved for usage by the FDA in March 2019, ketamine has been a glimpse of hope to patients with treatment-resistant MDD due to its rapid efficacy and relatively less adverse effects in comparison to other antidepressant medications. This has proved some relief to the patients, their families, clinicians and world governments’ healthcare budgets as well as to the economic burden that treating patients with depression causes to healthcare institutes. However, this interest should not become absolute as the studies that were made for testing ketamine are relatively small and some of the studies made showed considerable risks for abuse and addiction. In addition, ketamine had been shown to cause many side effects in multiple cases. Further studies should be made focused on understanding more about the mechanism of action of ketamine, as most of the studies differ quite significantly on how ketamine acts on depression. They should also focus on limiting the potential risk of addiction that ketamine carries. That could further help in increasing the efficacy and limiting the adverse effects of ketamine.
References
Abdullah Mohammed Hassan Ramadan is a 6th year Medical Student in Mansoura Manchester Program for Medical Education, University of Mansoura in Egypt. His main research interests include neurotransmitter receptors and their functions in drugs with different mechanisms of action, and medications for depression.

Footnotes
Contributors Both authors only have contributed to the work published.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.