Article Text
Abstract
Background Attention-deficit/hyperactivity disorder (ADHD) is a common behavioural disorder in childhood. The psychostimulant methylphenidate hydrochloride (MPH) is one of the major pharmacological options for ADHD. MPH is known to result, on average, in a small increase in arterial blood pressure (BP). However, there are few clinical data regarding the individual influences of MPH on BP among children and adolescents with ADHD. According to the European Union-wide standardised patient information sheet for MPH, BP changes >10 mm Hg compared with baseline values are ‘common’ (ie, ≥1% to <10%) in children and adolescents with ADHD during MPH therapy.
Aim To investigate the frequency and individual severity of BP changes in children and adolescents with ADHD during the first 6 months of new MPH therapy.
Methods In this study, 44 (77% male) children and adolescents (mean age (SD) 9.13 (1.86) years) with a diagnosis of ADHD according to the International Classification of Diseases, tenth revision, underwent ambulatory BP monitoring before and during the first 6 months of routine MPH therapy. Exclusion criteria were pre-existing MPH therapy and other medications that potentially influence BP or interfere with MPH. The non-interventional study was conducted prospectively at 10 paediatric cardiology centres in Germany and Austria.
Results After beginning MPH therapy, 34% of participants (99% CI 15.52% to 52.66%) had BP increases/decreases >10 mm Hg. The mean changes in systolic BP and diastolic BP were 0.87 mm Hg (95% CI -1.75 mm Hg to 3.48 mm Hg) and 1.96 mm Hg (95% CI 0.21 mm Hg to 3.7 mm Hg), respectively. The proportion of participants with initial prehypertension/hypertension was 54.55% .
Conclusions In our sample with a high baseline rate of prehypertension/hypertension, BP changes >10 mm Hg during MPH therapy were more frequent than those indicated by the patient information sheet. Moreover, individual BP changes, including increases and decreases >10 mm Hg, resulted in a small average BP increase in the sample, thus reflecting neither the severity nor the direction of individual BP changes. Therefore, the frequency and, due to the common use of the arithmetic mean, the individual severity of BP changes during MPH therapy may be underestimated. Further studies without averaging and with larger samples including patients in primary care settings are warranted.
- attention deficit disorder with hyperactivity
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is among the most common behavioural disorders in childhood and currently affects more than 63 million children and adolescents worldwide.1 The lack of concentration, hyperactivity, and occasional impulsive and antisocial behaviours of these children often result in sustained impairment of social, educational, and professional levels.2 Pharmacological treatment using the highly effective psychostimulant methylphenidate hydrochloride (MPH) is one of the main therapeutic options for ADHD.2 However, the ‘Special warnings and precautions’ subsection of the European Union (EU)-wide standardised patient information sheet regarding MPH warns that children and adolescents with ADHD commonly (ie, ≥1% to <10%) develop changes in systolic blood pressure (BP) and diastolic BP >10 mm Hg, compared with their baseline values, during MPH therapy.3 This warning is based on a comprehensive risk assessment of MPH-containing drugs commissioned by the European Commission from the European Medicines Agency in 2007.4 The need for this risk assessment was prompted by concerns regarding the extent of severe adverse events related to pharmacological MPH products after MPH-related cardiovascular events and strokes in Germany and other EU countries.5 Consequently, a detailed cardiovascular examination including BP measurements is recommended for every patient with ADHD before and during MPH therapy.3 4 However, pre-existing clinical data regarding the individual influences of MPH on BP among children and adolescents with ADHD are lacking. To our knowledge, only one small (n=8) study has addressed this issue,6 using ambulatory BP monitoring (ABPM) as the gold standard for measuring BP.7 A second study8 that focused on the individual influences of MPH on BP used less reliable single-point BP measurements. In contrast, other studies regarding this topic have reported average BP changes in the whole study group instead of individual BP changes.9–15 The method of averaging BP changes in all participants within a study group relativises stronger individual BP reactions, especially when there are BP changes in both directions, ie increases and decreases. Hence, the method of averaging BP changes might not permit inferences regarding the MPH load on the cardiovascular system at the level of the individual patient. These limitations underline the clinical importance of a systematic evaluation of individual BP changes during MPH treatment. Our observational study addressed the aforementioned limitations through the comparison of ABPM measurements performed before and during the first 6 months of new MPH therapy. An assessment of the frequency and individual severity of BP changes in children and adolescents with ADHD during MPH therapy is presented.
Methods
Participant recruiting process
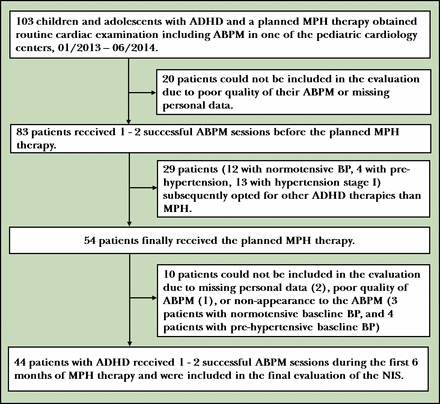
Patients with ADHD were recruited from nine paediatric cardiology centres in Germany and one in Austria. Inclusion criteria were age 6–13 years (defined as children) or 14–18 years (defined as adolescents);16 confirmed ADHD diagnosis according to the International Classification of Diseases, tenth revision;17 an individual decision to initiate MPH therapy; and written informed consent from the participants’ parents and from the participants themselves depending on their age. Exclusion criteria were pre-existing MPH therapy or use of other medications that could potentially influence BP or interfere with MPH. In accordance with the scope of practice of different medical specialists, the ADHD diagnosis and decisions regarding therapy were the responsibility of a child and youth psychiatrist. Detailed cardiac examinations, recommended before and during MPH therapy,3 were performed by a paediatric cardiologist. After the patients were referred by the psychiatrist to the cardiologist for routine cardiac examination, they were recruited by the latter according to the inclusion and exclusion criteria for the non-interventional study (NIS). Cardiologists measured BP and collected personal data relevant to the NIS. (figure 1)
Observation process. ABPM, ambulatory blood pressure monitoring; ADHD, attention-deficit/hyperactivity disorder; BP, blood pressure; MPH, methylphenidate hydrochloride; NIS, non-interventional study.
Sample size determination
All eligible patients at each of the study sites during the study period were included in the study group.
BP monitoring
ABPM is the gold standard for the diagnosis and monitoring of arterial hypertension in children and adolescents.7 The ABPM device (Mobil-O-Graph-NG; IEM GmbH, Stolberg, Germany) used for this study has been validated by the British Hypertension Society18 and the European Society of Hypertension,19 and is suitable for use in children.
Observation process
All participants completed a 24-hour ABPM session before the start of MPH therapy, thereby providing the baseline BP. A second session before the start of MPH therapy was intended (but not obligatory) to achieve familiarisation with the measurement method. Subsequently, a 24-hour ABPM session was conducted during the first 6 months of MPH therapy, with the measured BP compared with the baseline BP. A second session during MPH therapy was intended (but not obligatory) to increase the likelihood of monitoring possible BP changes during MPH therapy. If there were two ABPM sessions during MPH therapy, one was performed during the first 7 weeks after the start of MPH therapy and one was performed during the period thereafter when titration of the individual MPH dose was completed. The individual observation period ended 6 months after the start of MPH therapy.
BP measurement protocol
Children and adolescents, especially those with ADHD, tend to move during BP measurements, which might result in an incorrect BP measurement or render measurements impossible. To increase participant acceptance, measurements were reduced to a minimum of two per hour during the day and one per hour during the night. Daytime was defined from 08:00 to 20:00, and night-time was from 00:00 to 06:00. Time intervals between these two defined periods were discarded because of interindividual differences in sleep-wake cycles. A maximum of 32 single-point BP measurements within a 24-hour cycle was possible. Only ABPM sessions with at least six daytime measurements were eligible for further analyses. Additionally, at least four night-time measurements were mandatory for adolescents because night-time BP is needed to assess arterial hypertension in adolescents.20 Measurements beyond the following ranges were excluded: systolic BP, 60–220 mm Hg; diastolic BP, 35–120 mm Hg; and heart rate, 40–180 bpm.
MPH therapy
Because psychiatric treatment was based on the clinical practice of the responsible child and youth psychiatrist, participants were treated with different preparations of the active ingredient MPH. These included the following: Medikinet and Medikinet retard (MEDICE Arzneimittel Pütter GmbH & Co. KG; Iserlohn, Germany); Equasym retard (Shire Deutschland GmbH; Berlin, Germany); Ritalin LA (Novartis Pharma GmbH; Nürnberg, Germany); and Concerta retard (Janssen-Cilag GmbH; Neuss, Germany). As recommended by the patient information sheet, MPH therapy was started with the lowest possible dose, which was usually 5 mg of MPH once or twice per day.3 Then, the daily dose was increased at weekly intervals by 5–10 mg, depending on the individual participant’s response and tolerability. The mean daily MPH dose was 21.16 (11.14) mg and did not exceed 60 mg. MPH was administered as one, two or three doses per day.
Data analysis
For each individual, the measurements of each ABPM session were averaged separately for daytime and night-time. To obtain a more reliable baseline BP, based on the effect of a regression to the middle,21 averaging of baseline ABPM measurements was performed for cases where two sessions were conducted. Then, ABPM measurements during MPH therapy were compared with baseline values. In service of our aim of identifying the severity of BP changes during MPH therapy, when two ABPM sessions were performed during MPH therapy, the one with the greater BP effect was selected for analysis. Therefore, our analysis approach adequately reflected the potential cardiovascular load on the individual patient, which is crucial because even a single occurrence of a severe BP change can have acute clinical consequences.22 Because the EU-wide standardised patient information sheet for MPH estimates that BP changes >10 mm Hg are ‘common’ (≥1% to <10%),3 this information served as a comparative size for the study results. Accordingly, a relevant BP change was defined as a >10 mm Hg change (increase or decrease) in systolic BP or diastolic BP during either daytime or night-time; the ‘common’ appearance of the adverse effect was defined as stated previously. To improve the comparability of the results with those of other studies, parameters of descriptive statistics were calculated because existing studies of this topic mainly provide these data. For the sensitivity analysis, the 95% CI and 99% CI were calculated.
To enable interindividual analyses, SD scores (z-scores) for the daytime BP of all participants were calculated using the LMS method (with L, skewness; M, median; and S, coefficient of variation) described by Cole and Green23 and modified by Neuhauser et al.24 Hereby, participants’ BP results were transformed into the value range of the normal distribution of a healthy reference population, standardised by age, sex, and height. The population of the German Health Interview and Examination Survey for Children and Adolescents was chosen as the reference group.24 Then, participants were categorised into five groups according to their z-scores, following the German guideline for arterial hypertension20 and the recommendations of the European Society of Hypertension7 (table 1): hypotension, normotension, prehypertension, hypertension stage I, and hypertension stage II. The individual BP group at baseline was compared with the BP group during MPH therapy. When two ABPM sessions were performed during MPH therapy, the one with the greater change in the BP category (eg, from normotension to hypertension) was chosen for data analyses, which was consistent with our aim of measuring the maximum possible individual load of MPH on the cardiovascular system.
Definition of arterial hypertension for ambulatory BP monitoring (ABPM) in children and adolescents
Efforts to address potential sources of bias
For quality assurance, all participating paediatric cardiologists used the same ABPM device. All ABPM devices were programmed according to the study protocol and were installed by trained staff. Moreover, measurements per day were reduced to a minimum to increase participants’ acceptance. Higher BP values that might be caused by a high activity level during ABPM were controlled for by averaging the multiple ABPM measurements within one session (effect of regression to the middle). We excluded participants with a history of MPH therapy and those using other medications that might interact with MPH or influence BP.
Patient involvement
BP measurements obtained before and during MPH therapy are part of routine care for children and adolescents with ADHD. In this NIS, ABPM was chosen to measure BP because it is the gold standard.7 Nevertheless, ABPM can be more stressful for participants than the alternative single-point BP measurements. Therefore, participants and their parents were informed about the advantages and disadvantages of ABPM measurements, and participants were asked to assess the burden of using ABPM, including the imposed stress and required time.
Reporting guidelines
The Strengthening the Reporting of Observational Studies in Epidemiology Statement was used for reporting the NIS.
Results
Participants
Forty-four children and adolescents successfully participated in the NIS. Figure 1 shows the details of the individuals at each stage of the study (eg, numbers of those included in the study, those who received the planned MPH therapy, and those who completed follow-up). Demographic data of the participants are presented in table 2.
Demographic data of the study population (n=44)
BP results
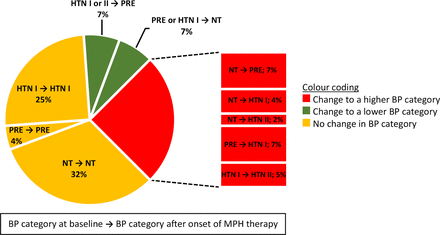
BP changes >10 mm Hg were observed in 15 of the 44 participants (34.09%; 99% CI 15.52% to 52.66%) after the start of MPH therapy. BP results are presented in online supplementary table 1, and detailed systolic BP and diastolic BP changes are shown in online supplementary table 2. As illustrated in figure 2, 54.55% (95% CI 39.61% to 69.49%) of the 44 participants was prehypertensive or hypertensive prior to MPH therapy. For 22 of these 24 prehypertensive or hypertensive participants, BP was calculated as the mean of two ABPM sessions. One of the 24 prehypertensive or hypertensive participants was overweight and four were obese. In the normotensive group (n=20), three participants were overweight or obese. Table 3 summarises the descriptive statistics.
Supplemental material
Supplemental material
Descriptive statistics for daytime BP changes during MPH therapy
Change in BP category after onset of MPH therapy (n=44). BP, blood pressure; HTN I or II, hypertensive blood pressure stage I or II; MPH, methylphenidate hydrochloride; NT, normotensive blood pressure; PRE, prehypertensive blood pressure.
Discussion
Main findings
Two main observations were made regarding the frequency and individual severity of BP changes during the first 6 months of new MPH therapy. First, BP changes >10 mm Hg were observed in 34.09% (99% CI 15.52% to 52.66%) of the 44 participants after the start of MPH therapy. The EU-wide standardised patient information sheet for MPH estimates that BP changes >10 mm Hg are ‘common’ (≥1% to <10%) in children and adolescents with ADHD treated with MPH.3 Consequently, in our sample of children and adolescents with a high baseline rate of prehypertension and hypertension, the occurrence of BP changes >10 mm Hg during MPH therapy was higher than what was stated by the patient information sheet. To investigate the possibility that the frequency of BP changes during MPH therapy is underestimated, further studies with larger samples that include children in primary care settings are warranted. Second, the individual BP changes observed in the 44 participants considerably varied on both severity and direction, including BP increases and BP decreases >10 mm Hg. These individual BP changes resulted in average BP changes of 0.87 mm Hg (95% CI -1.75 mm Hg to 3.48 mm Hg) and 1.96 mm Hg (95% CI 0.21 mm Hg to 3.7 mm Hg) in systolic daytime BP and diastolic daytime BP, respectively. However, these relatively small average BP increases in the sample reflect neither the severity nor the direction of individual BP changes because the use of the arithmetic mean naturally neutralises opposing BP changes and relativises stronger individual BP reactions. Nevertheless, previous relevant studies usually used the arithmetic mean to measure the severity of BP changes during MPH therapy in children and adolescents with ADHD. These studies reported results similar to those of this NIS with an average BP increase in the sample of 1–4 mm Hg in systolic daytime BP and/or diastolic daytime BP.9–15 A recent meta-analysis confirmed a statistically significant increase in the averaged post-treatment versus pretreatment systolic BP as compared with placebo.25 It is possible that the BP results of these studies that reported statistically significant but clinically ‘small’,8 14 ‘slight’,13 ‘minimal’,10 or ‘minor’15 BP changes in children and adolescents with ADHD might have been stronger if the arithmetic mean had not been used. Consequently, the common use of the arithmetic mean potentially led to an underestimation of the severity of BP changes during MPH therapy. To investigate this possibility, further research without averaging seems warranted. This is important for the individual patient because information about the possibility or, even better, the frequency of strong BP increases and decreases during MPH therapy is clinically more crucial than information about the average BP change in a sample. An increase or decrease in BP is associated with different clinical consequences, depending on the patient risk group. For example, congestive heart failure, hypertensive encephalopathy, or renal failure may occur as a result of an acute BP increase.22 Lower BP in younger individuals is associated with an increased incidence of accidental deaths, which is possibly an indication of reduced reaction performance at low BP.26 Finally, a single severe BP change can have acute clinical consequences,22 especially in patients with cardiovascular predispositions.
Additional findings
BP changes can lead to changes in the BP category, which is used to determine the initiation of diagnostic and therapeutic measures according to the guidelines.27 Because of BP increases/decreases during MPH therapy, 11 of 44 participants (25%) were categorised into a higher BP group than they had previously been categorised in (eg, normotension to hypertension), whereas 6 participants (14%) were categorised into a lower BP group (figure 2). Similar results (although based on single-point BP measurements) were shown by Stowe et al6 and Hammerness et al,8 who reported newly met criteria for prehypertension or hypertension in 37.5% of 8 patients and 14.04% of 114 patients with ADHD, respectively, after the beginning of MPH therapy. Stowe et al6 also reported a patient with initial hypertensive BP values based on single BP measurements who additionally met the ABPM criteria of arterial hypertension during MPH therapy. However, BP classification during childhood and adolescence, unlike the BP classification in adulthood, is based on the statistical normal distribution of the BP values in a healthy comparison group.20 21 24 Accordingly, exceeding or falling below the percentile limits for normotension is not necessarily associated with cardiovascular consequences (although, an association between statistically defined ‘high’ BP levels in childhood, and high BP values and their corresponding cardiovascular consequences in adulthood is well known28). Additionally, the percentile-oriented interpretation of BP is ordinally scaled and shows a very broad normal range (5th to 90th percentiles of the reference group), but with only very narrow prehypertensive, hypertensive and hypotensive BP ranges20 21 24 that are difficult to reach. Consequently, a change in the BP category and as well an absolute BP change in mm Hg should be considered during the risk-benefit assessment of MPH therapy.
Interestingly, the proportion of participants with baseline prehypertension or hypertension was relatively high. According to the percentile-dependent definition of arterial prehypertension and hypertension during childhood, only 10% of the study participants were expected to have elevated BP values (ie, ≥90th percentile) before the beginning of MPH therapy. However, the proportion of prehypertensive or hypertensive participants in the NIS was 54.55% (95% CI 39.61% to 69.49%). The study by Hammerness et al8 showed similar results, with 29% of 114 participants having arterial hypertension. Therefore, systematically increased BP levels in children and adolescents with ADHD seem likely. A connection between neurocognitive disorders, such as ADHD, and arterial hypertension is possible.29 Although the question of a causal relationship between neurocognitive disorders and arterial hypertension cannot be answered with this study design, the findings of this study encourage further investigations into arterial hypertension as a possible cause or consequence of ADHD. However, selection bias as the cause of the high proportion of initially prehypertensive or hypertensive participants in the NIS cannot be ruled out. Overall, the high baseline rate of prehypertension and hypertension observed in patients with ADHD is worth mentioning because screening for cardiac disorders before stimulant therapy is frequently disregarded. According to a survey of 615 members of the American Academy of Child and Adolescent Psychiatry, up to 25% of respondents do not routinely perform the officially recommended physical examination for their patients before stimulant therapy.30 In these cases, the patients’ cardiovascular predispositions, eg severe arterial hypertension as a contraindication for MPH therapy,3 remain undetected. This emphasizes the importance of the recommended cardiovascular examination of every ADHD patient before MPH therapy.
Limitations
The external validity of the study sample has certain limitations. Given the high proportion of participants with pre-existing prehypertension or hypertension, BP values measured during MPH therapy may not be representative of patients with ADHD without such a cardiovascular risk profile. Furthermore, BP values collected before the start of MPH therapy apply to children and adolescents whose ADHD symptom severity had already led to psychiatric presentation and the planning of stimulant therapy. Therefore, these results may not be representative of children and adolescents with only mild ADHD who do not require drug therapy.
Although the psychiatrists near the cardiology centres were familiarised with the study design, it cannot be ruled out that they referred predominantly patients with ADHD with high-risk cardiovascular profiles to paediatric cardiology centres. This would have resulted in channeling bias, and might then partly explain the high number of prehypertensive or hypertensive participants in the sample. Because the total number of patients treated with MPH by psychiatrists was not documented, the degree of selection bias in the sample was difficult to estimate.
In favour of epidemiologically appropriate BP percentiles, methodological differences regarding the BP measurement method were accepted. Neuhauser et al24 used two single-point BP measurements, whereas the present study chose ABPM. Nevertheless, serious inaccuracies in percentile classifications are rather unlikely because resting daytime BP in children and adolescents measured using an ambulatory method or in clinical practice does not differ remarkably.7 Furthermore, higher ambulatory BP values that might be caused by daily activity during ABPM are relativised by averaging the multiple measurements of the ABPM session (effect of the regression to the middle).
Because the follow-up period was only 6 months, the long-term haemodynamic effects during MPH therapy and the impact of these effects on adults formerly treated with MPH could not be addressed. A study with long-term follow-up is needed to answer these questions.
Implications
In our sample with a high baseline rate of prehypertension and hypertension, BP changes >10 mm Hg during MPH therapy were more frequent than those indicated by the EU-wide standardised patient information sheet. Moreover, individual BP changes, including BP increases and decreases >10 mm Hg, considerably varied. These individual BP changes resulted in a small average BP increase in the sample. Thus, the arithmetic mean reflected neither the severity nor the direction of individual BP changes because the use of the arithmetic mean neutralises opposing BP changes and relativizes stronger individual BP reactions. In spite of this, the arithmetic mean is commonly used to indicate the severity of the possible BP change during MPH therapy. Therefore, the frequency and individual severity of BP changes during MPH therapy may be underestimated. Further studies without averaging and with larger samples including patients in primary care settings are warranted.
Abstract translation
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.Acknowledgments
The authors thank the study sites for collecting the data and providing and taking care of the study participants.
References
Dorothee Busold-Hagenbeck studied medicine at the University of Göttingen in Germany and obtained her license to practise in 2016. In 2017, she obtained a Bachelor of Arts in Philosophy and Religious Studies from the University of Göttingen to pursue her interest in the perception of health and illness according to various religions and philosophical traditions. She was awarded a scholarship from the state of Lower Saxony for extraordinary academic achievements, outstanding qualifications, and outstanding honorary commitments. Dorothee gained work experience in the fields of clinical psychiatry and psychotherapy as well as in general internal medicine. Currently, she works as an assistant internal medicine physician in a hospital in Berlin, Germany. In her dissertation project, she investigates the influence of the drug methylphenidate hydrochloride on the blood pressure of children and adolescents with attention-deficit/hyperactivity disorder.

Footnotes
Presented at This work was previously presented as a work-in-progress with preliminary findings at a conference and was published as a conference abstract: Hulpke-Wette M, Hagenbeck D, Irtel von Brenndorff C. Anwendungsbeobachtung zur Erfassung möglicher Blutdruckveränderungen bei Kindern und Jugendlichen mit Aufmerksamkeitsdefizit-Hyperaktivitäts-Syndrom (ADHS) unter Methylphenidat-Therapie mit Hilfe von 24-Stunden Langzeitblutdruckmessungen. Thorac Cardiovasc Surg 2016;64(S02):ePP13. doi:10.1055/s-0036–1571915.
Contributors DB-H, MH-W, RH and CIvB contributed to study conception and design and data acquisition. DB-H performed the statistical analysis. JE and RH contributed to the statistical analysis plan and the data analysis. DB-H wrote the manuscript. JE contributed to writing the manuscript. All authors contributed to interpreting the analysis and critically revising the article, approved the final draft and agreed to be accountable for all aspects of the work. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MH-W is the guarantor.
Funding The study was partly funded by the German Working Group of Paediatric Cardiologists in Private Practice (ANKK e. V.), which funded governmental registration fees and office supplies. The provision of the ABPM devices at the time of the observational process by the manufacturer IEM GmbH included the devices itself and the patient management software.
Competing interests DB-H, MH-W and CIvB report grants from the German Working Group of Paediatric Cardiologists in Private Practice (ANKK e. V.) and non-financial support from IEM GmbH during the conduct of the study (see the ‘Funding’ section above).
Patient consent for publication Written informed consent for publication was obtained from the participants’ parents and from the participants themselves depending on their age.
Ethics approval The study was conducted in accordance with the ethical principles of World Medical Association’s Declaration of Helsinki and was approved by the ethical committee of the University of Göttingen (ID: 15/7/12).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.